IL6 Mediates Suppression of T- and NK-cell Function in EMT-associated TKI-resistant EGFR-mutant NSCLC
- PMID: 36595561
- PMCID: PMC10290888
- DOI: 10.1158/1078-0432.CCR-22-3379
IL6 Mediates Suppression of T- and NK-cell Function in EMT-associated TKI-resistant EGFR-mutant NSCLC
Abstract
Purpose: Patients with advanced non-small cell lung cancer (NSCLC) harboring activating EGFR mutations are initially responsive to tyrosine kinase inhibitors (TKI). However, therapeutic resistance eventually emerges, often via secondary EGFR mutations or EGFR-independent mechanisms such as epithelial-to-mesenchymal transition. Treatment options after EGFR-TKI resistance are limited as anti-PD-1/PD-L1 inhibitors typically display minimal benefit. Given that IL6 is associated with worse outcomes in patients with NSCLC, we investigate whether IL6 in part contributes to this immunosuppressed phenotype.
Experimental design: We utilized a syngeneic genetically engineered mouse model (GEMM) of EGFR-mutant NSCLC to investigate the effects of IL6 on the tumor microenvironment and the combined efficacy of IL6 inhibition and anti-PD-1 therapy. Corresponding in vitro studies used EGFR-mutant human cell lines and clinical specimens.
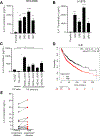
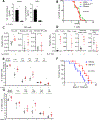
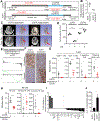
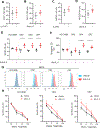
Results: We identified that EGFR-mutant tumors which have oncogene-independent acquired resistance to EGFR-TKIs were more mesenchymal and had markedly enhanced IL6 secretion. In EGFR-mutant GEMMs, IL6 depletion enhanced activation of infiltrating natural killer (NK)- and T-cell subpopulations and decreased immunosuppressive regulatory T and Th17 cell populations. Inhibition of IL6 increased NK- and T cell-mediated killing of human osimertinib-resistant EGFR-mutant NSCLC tumor cells in cell culture. IL6 blockade sensitized EGFR-mutant GEMM tumors to PD-1 inhibitors through an increase in tumor-infiltrating IFNγ+ CD8+ T cells.
Conclusions: These data indicate that IL6 is upregulated in EGFR-mutant NSCLC tumors with acquired EGFR-TKI resistance and suppressed T- and NK-cell function. IL6 blockade enhanced antitumor immunity and efficacy of anti-PD-1 therapy warranting future clinical combinatorial investigations.
©2023 American Association for Cancer Research.
Conflict of interest statement
The remaining authors declare no conflicts of interest.
Figures
References
-
- Gao J, et al., Strategies to overcome acquired resistance to EGFR TKI in the treatment of non-small cell lung cancer. Clin Transl Oncol, 2019. 21(10): p. 1287–1301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

