Influence of Genomic Landscape on Cancer Immunotherapy for Newly Diagnosed Ovarian Cancer: Biomarker Analyses from the IMagyn050 Randomized Clinical Trial
- PMID: 36595569
- PMCID: PMC10150250
- DOI: 10.1158/1078-0432.CCR-22-2032
Influence of Genomic Landscape on Cancer Immunotherapy for Newly Diagnosed Ovarian Cancer: Biomarker Analyses from the IMagyn050 Randomized Clinical Trial
Abstract
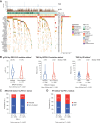
Purpose: To explore whether patients with BRCA1/2-mutated or homologous recombination deficient (HRD) ovarian cancers benefitted from atezolizumab in the phase III IMagyn050 (NCT03038100) trial.
Patients and methods: Patients with newly diagnosed ovarian cancer were randomized to either atezolizumab or placebo with standard chemotherapy and bevacizumab. Programmed death-ligand 1 (PD-L1) status of tumor-infiltrating immune cells (IC) was determined centrally (VENTANA SP142 assay). Genomic alterations, including deleterious BRCA1/2 alterations, genomic loss of heterozygosity (gLOH), tumor mutation burden (TMB), and microsatellite instability (MSI), were evaluated using the FoundationOne assay. HRD was defined as gLOH ≥ 16%, regardless of BRCA1/2 mutation status. Potential associations between progression-free survival (PFS) and genomic biomarkers were evaluated using standard correlation analyses and log-rank of Kaplan-Meier estimates.
Results: Among biomarker-evaluable samples, 22% (234/1,050) harbored BRCA1/2 mutations and 46% (446/980) were HRD. Median TMB was low irrespective of BRCA1/2 or HRD. Only 3% (29/1,024) had TMB ≥10 mut/Mb, and 0.3% (3/1,022) were MSI-high. PFS was better in BRCA2-mutated versus BRCA2-non-mutated tumors and in HRD versus proficient tumors. PD-L1 positivity (≥1% expression on ICs) was associated with HRD but not BRCA1/2 mutations. PFS was not improved by adding atezolizumab in BRCA2-mutated or HRD tumors; there was a trend toward enhanced PFS with atezolizumab in BRCA1-mutated tumors.
Conclusions: Most ovarian tumors have low TMB despite BRCA1/2 mutations or HRD. Neither BRCA1/2 mutation nor HRD predicted enhanced benefit from atezolizumab. This is the first randomized double-blind trial in ovarian cancer demonstrating that genomic instability triggered by BRCA1/2 mutation or HRD is not associated with improved sensitivity to immune checkpoint inhibitors. See related commentary by Al-Rawi et al., p. 1645.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures



Comment in
-
The Search for Genomic Biomarkers of Response to Immunotherapy in Ovarian Cancer.Clin Cancer Res. 2023 May 1;29(9):1645-1647. doi: 10.1158/1078-0432.CCR-23-0048. Clin Cancer Res. 2023. PMID: 36826422 Free PMC article.
Comment on
-
The Search for Genomic Biomarkers of Response to Immunotherapy in Ovarian Cancer.Clin Cancer Res. 2023 May 1;29(9):1645-1647. doi: 10.1158/1078-0432.CCR-23-0048. Clin Cancer Res. 2023. PMID: 36826422 Free PMC article.
References
-
- Pujade-Lauraine E, Fujiwara K, Ledermann JA, Oza AM, Kristeleit R, Ray-Coquard I-L, et al. Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN Ovarian 200): an open-label, three-arm, randomized, phase III study. Lancet Oncol 2021;22:1034–46. - PubMed
-
- Monk BJ, Colombo N, Oza AM, Fujiwara K, Birrer MJ, Randall L, et al. Chemotherapy with or without avelumab followed by avelumab maintenance versus chemotherapy alone in patients with previously untreated epithelial ovarian cancer (JAVELIN Ovarian 100): an open-label, randomized, phase III trial. Lancet Oncol 2021;22:1275–89. - PubMed
-
- Vivot A, Créquit P, Porcher R. Use of late-life expectancy for assessing the long-term benefit of immune checkpoint inhibitors. J Natl Cancer Inst 2019;111:519–21. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

