Dry eye disease in mice activates adaptive corneal epithelial regeneration distinct from constitutive renewal in homeostasis
- PMID: 36595669
- PMCID: PMC9926235
- DOI: 10.1073/pnas.2204134120
Dry eye disease in mice activates adaptive corneal epithelial regeneration distinct from constitutive renewal in homeostasis
Abstract
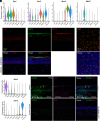
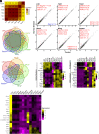
Many epithelial compartments undergo constitutive renewal in homeostasis but activate unique regenerative responses following injury. The clear corneal epithelium is crucial for vision and is renewed from limbal stem cells (LSCs). Using single-cell RNA sequencing, we profiled the mouse corneal epithelium in homeostasis, aging, diabetes, and dry eye disease (DED), where tear deficiency predisposes the cornea to recurrent injury. In homeostasis, we capture the transcriptional states that accomplish continuous tissue turnover. We leverage our dataset to identify candidate genes and gene networks that characterize key stages across homeostatic renewal, including markers for LSCs. In aging and diabetes, there were only mild changes with <15 dysregulated genes. The constitutive cell types that accomplish homeostatic renewal were conserved in DED but were associated with activation of cell states that comprise "adaptive regeneration." We provide global markers that distinguish cell types in homeostatic renewal vs. adaptive regeneration and markers that specifically define DED-elicited proliferating and differentiating cell types. We validate that expression of SPARC, a marker of adaptive regeneration, is also induced in corneal epithelial wound healing and accelerates wound closure in a corneal epithelial cell scratch assay. Finally, we propose a classification system for LSC markers based on their expression fidelity in homeostasis and disease. This transcriptional dissection uncovers the dramatically altered transcriptional landscape of the corneal epithelium in DED, providing a framework and atlas for future study of these ocular surface stem cells in health and disease.
Keywords: cornea; dry eye; epithelium; limbal; stem cell.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

