Anatomically interpretable deep learning of brain age captures domain-specific cognitive impairment
- PMID: 36595679
- PMCID: PMC9926270
- DOI: 10.1073/pnas.2214634120
Anatomically interpretable deep learning of brain age captures domain-specific cognitive impairment
Abstract
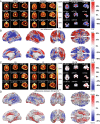
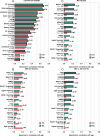
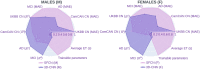
The gap between chronological age (CA) and biological brain age, as estimated from magnetic resonance images (MRIs), reflects how individual patterns of neuroanatomic aging deviate from their typical trajectories. MRI-derived brain age (BA) estimates are often obtained using deep learning models that may perform relatively poorly on new data or that lack neuroanatomic interpretability. This study introduces a convolutional neural network (CNN) to estimate BA after training on the MRIs of 4,681 cognitively normal (CN) participants and testing on 1,170 CN participants from an independent sample. BA estimation errors are notably lower than those of previous studies. At both individual and cohort levels, the CNN provides detailed anatomic maps of brain aging patterns that reveal sex dimorphisms and neurocognitive trajectories in adults with mild cognitive impairment (MCI, N = 351) and Alzheimer's disease (AD, N = 359). In individuals with MCI (54% of whom were diagnosed with dementia within 10.9 y from MRI acquisition), BA is significantly better than CA in capturing dementia symptom severity, functional disability, and executive function. Profiles of sex dimorphism and lateralization in brain aging also map onto patterns of neuroanatomic change that reflect cognitive decline. Significant associations between BA and neurocognitive measures suggest that the proposed framework can map, systematically, the relationship between aging-related neuroanatomy changes in CN individuals and in participants with MCI or AD. Early identification of such neuroanatomy changes can help to screen individuals according to their AD risk.
Keywords: Alzheimer’s disease; brain age; cognitive impairment; deep learning.
Conflict of interest statement
The authors have research support to disclose, P.M.T. discloses research grant support from Biogen, Inc. for work unrelated to this study.
Figures

References
Publication types
MeSH terms
Grants and funding
- R01 AG058854/AG/NIA NIH HHS/United States
- P41 EB015922/EB/NIBIB NIH HHS/United States
- R01 NS100973/NS/NINDS NIH HHS/United States
- U24 AG021886/AG/NIA NIH HHS/United States
- U01 AG024904/AG/NIA NIH HHS/United States
- U01 AG068057/AG/NIA NIH HHS/United States
- RF1 AG082201/AG/NIA NIH HHS/United States
- BB/H008217/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- U01 AG052564/AG/NIA NIH HHS/United States
- U54 MH091657/MH/NIMH NIH HHS/United States
- R01 AG079957/AG/NIA NIH HHS/United States
- R01 AG060610/AG/NIA NIH HHS/United States
- MC_PC_17228/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

