A bioengineered probiotic for the oral delivery of a peptide Kv1.3 channel blocker to treat rheumatoid arthritis
- PMID: 36595694
- PMCID: PMC9926172
- DOI: 10.1073/pnas.2211977120
A bioengineered probiotic for the oral delivery of a peptide Kv1.3 channel blocker to treat rheumatoid arthritis
Abstract
Engineered microbes for the delivery of biologics are a promising avenue for the treatment of various conditions such as chronic inflammatory disorders and metabolic disease. In this study, we developed a genetically engineered probiotic delivery system that delivers a peptide to the intestinal tract with high efficacy. We constructed an inducible system in the probiotic Lactobacillus reuteri to secrete the Kv1.3 potassium blocker ShK-235 (LrS235). We show that LrS235 culture supernatants block Kv1.3 currents and preferentially inhibit human T effector memory (TEM) lymphocyte proliferation in vitro. A single oral gavage of healthy rats with LrS235 resulted in sufficient functional ShK-235 in the circulation to reduce inflammation in a delayed-type hypersensitivity model of atopic dermatitis mediated by TEM cells. Furthermore, the daily oral gavage of LrS235 dramatically reduced clinical signs of disease and joint inflammation in rats with a model of rheumatoid arthritis without eliciting immunogenicity against ShK-235. This work demonstrates the efficacy of using the probiotic L. reuteri as a novel oral delivery platform for the peptide ShK-235 and provides an efficacious strategy to deliver other biologics with great translational potential.
Keywords: Kv1.3 channel; drug delivery; synthetic biology.
Conflict of interest statement
The authors declare competing interest. The authors have organizational affiliations to disclose. R.A.B. is a cofounder of Mikrovia and PanaBio. The authors have patent filings to disclose. C.B. and M.W.P. are inventors on the patent protecting ShK-186, currently in clinical trials for the treatment of autoimmune diseases. C.B., J.M.H., and R.A.B. are inventors on a patent disclosure protecting LrS235.
Figures
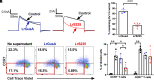
 ) or LrS235 (
) or LrS235 ( ) supernatants diluted 1/10. Mean ± SEM, each data point represents a different measurement. (C) Representative flow cytometry plots of CellTrace Violet dye dilution and CCR7 expression of CD3+ cells from human peripheral blood MNC stimulated for 7 d without any bacterial supernatants (Left) or in the presence of supernatants from LrGusA (Middle) or LrS235 (Right). (D) Percent of divided human CCR7− TEM and CCR7+ naïve/TCM cells in the absence of stimulation (
) supernatants diluted 1/10. Mean ± SEM, each data point represents a different measurement. (C) Representative flow cytometry plots of CellTrace Violet dye dilution and CCR7 expression of CD3+ cells from human peripheral blood MNC stimulated for 7 d without any bacterial supernatants (Left) or in the presence of supernatants from LrGusA (Middle) or LrS235 (Right). (D) Percent of divided human CCR7− TEM and CCR7+ naïve/TCM cells in the absence of stimulation ( ) and after anti-CD3 induced stimulation in the presence of Lr medium (■) or supernatants of LrGusA (
) and after anti-CD3 induced stimulation in the presence of Lr medium (■) or supernatants of LrGusA ( ) or LrS235 (
) or LrS235 ( ) diluted 1/100. Mean ± SEM, N = 4 different buffy coat donors. *P < 0.05, **P < 0.01.
) diluted 1/100. Mean ± SEM, N = 4 different buffy coat donors. *P < 0.05, **P < 0.01.
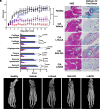
 ) every other day starting disease onset, 1 × 109 cfu LrGusA (
) every other day starting disease onset, 1 × 109 cfu LrGusA ( ) or LrS235 gavage daily (
) or LrS235 gavage daily ( ). B. Hematoxylin and eosin (Left) and safranin O/fast green (Right) staining and histology scoring (C) of joints from paws from CIA rats received different treatments. Original magnification, 10×, scale bars, 100 μm. Refer to “Histology and micro-CT” in the Materials and Methods for more details of the scoring system. D. Representative micro-CT of paws from CIA rats treated with vehicle, synthetic ShK-235 every other day, or oral gavage with LrGusA, LrS235 daily. Data presented as mean ± SEM. N = 7 to 10 rats per group. Asterisks indicate areas of cartilage erosions. *P < 0.05; **P < 0.01, ***P < 0.001, and ****P < 0.0001.
). B. Hematoxylin and eosin (Left) and safranin O/fast green (Right) staining and histology scoring (C) of joints from paws from CIA rats received different treatments. Original magnification, 10×, scale bars, 100 μm. Refer to “Histology and micro-CT” in the Materials and Methods for more details of the scoring system. D. Representative micro-CT of paws from CIA rats treated with vehicle, synthetic ShK-235 every other day, or oral gavage with LrGusA, LrS235 daily. Data presented as mean ± SEM. N = 7 to 10 rats per group. Asterisks indicate areas of cartilage erosions. *P < 0.05; **P < 0.01, ***P < 0.001, and ****P < 0.0001.
References
-
- Anselmo A. C., Gokarn Y., Mitragotri S., Non-invasive delivery strategies for biologics. Nat. Rev. Drug. Discov. 18, 19–40 (2019). - PubMed
-
- Smolen J. S., Aletaha D., McInnes I. B., Rheumatoid arthritis. Lancet 388, 2023–2038 (2016). - PubMed
-
- Voskuyl A. E., The heart and cardiovascular manifestations in rheumatoid arthritis. Rheumatology 45, 4–7 (2006). - PubMed
-
- van den Bemt B. J., Zwikker H. E., van den Ende C. H., Medication adherence in patients with rheumatoid arthritis: A critical appraisal of the existing literature. Expert Rev. Clin. Immunol. 8, 337–351 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

