Generation of human elongating multi-lineage organized cardiac gastruloids
- PMID: 36595961
- PMCID: PMC9727145
- DOI: 10.1016/j.xpro.2022.101898
Generation of human elongating multi-lineage organized cardiac gastruloids
Abstract
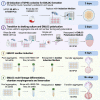
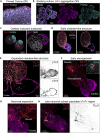
Human elongating multi-lineage organized (EMLOC) gastruloid technology captures key aspects of trunk neurodevelopment including neural integration with cardiogenesis. We generate multi-chambered, contractile EMLOC gastruloids with integrated central and peripheral neurons using defined culture conditions and signaling factors. hiPSC colonies are primed by activating FGF and Wnt signaling pathways for co-induced lineages. EMLOC gastruloids are then initialized with primed cells in suspension culture using timed exposure to FGF2, HGF, IGF1, and Y-27632. Cardiogenesis is stimulated by FGF2, VEGF, and ascorbic acid. For complete details on the use and execution of this protocol, please refer to Olmsted and Paluh (2022).1.
Keywords: Bioinformatics; Developmental biology; Neuroscience; Organoids; Stem Cells.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests Application for patent for which J.L.P. and Z.T.O. are co-inventors has been filed by the State University of New York Research Foundation (SUNYRF) with the US Patent Office on the EMLOC technology and detailed methods. U.S. patent filings: 63/311,498 and 63/419,507.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

