Analysis of a Biopsy-Based Genomic Classifier in High-Risk Prostate Cancer: Meta-Analysis of the NRG Oncology/Radiation Therapy Oncology Group 9202, 9413, and 9902 Phase 3 Randomized Trials
- PMID: 36596347
- PMCID: PMC10281690
- DOI: 10.1016/j.ijrobp.2022.12.035
Analysis of a Biopsy-Based Genomic Classifier in High-Risk Prostate Cancer: Meta-Analysis of the NRG Oncology/Radiation Therapy Oncology Group 9202, 9413, and 9902 Phase 3 Randomized Trials
Abstract
Purpose: Decipher is a genomic classifier (GC) prospectively validated postprostatectomy. We validated the performance of the GC in pretreatment biopsy samples within the context of 3 randomized phase 3 high-risk definitive radiation therapy trials.
Methods and materials: A prespecified analysis plan (NRG-GU-TS006) was approved to obtain formalin-fixed paraffin-embedded tissue from biopsy specimens from the NRG biobank from patients enrolled in the NRG/Radiation Therapy Oncology Group (RTOG) 9202, 9413, and 9902 phase 3 randomized trials. After central review, the highest-grade tumors were profiled on clinical-grade whole-transcriptome arrays and GC scores were obtained. The primary objective was to validate the independent prognostic ability for the GC for distant metastases (DM), and secondary for prostate cancer-specific mortality (PCSM) and overall survival (OS) with Cox univariable and multivariable analyses.
Results: GC scores were obtained on 385 samples, of which 265 passed microarray quality control (69%) and had a median follow-up of 11 years (interquartile range, 9-13). In the pooled cohort, on univariable analysis, the GC was shown to be a prognostic factor for DM (per 0.1 unit; subdistribution hazard ratio [sHR], 1.29; 95% confidence interval [CI], 1.18-1.41; P < .001), PCSM (sHR, 1.28; 95% CI, 1.16-1.41; P < .001), and OS (hazard ratio [HR], 1.16; 95% CI, 1.08-1.22; P < .001). On multivariable analyses, the GC (per 0.1 unit) was independently associated with DM (sHR, 1.22; 95% CI, 1.09-1.36), PCSM (sHR, 1.23; 95% CI, 1.09-1.39), and OS (HR, 1.12; 95% CI, 1.05-1.20) after adjusting for age, Prostate Specific Antigen, Gleason score, cT stage, trial, and randomized treatment arm. GC had similar prognostic ability in patients receiving short-term or long-term androgen-deprivation therapy, but the absolute improvement in outcome varied by GC risk.
Conclusions: This is the first validation of a gene expression biomarker on pretreatment prostate cancer biopsy samples from prospective randomized trials and demonstrates an independent association of GC score with DM, PCSM, and OS. High-risk prostate cancer is a heterogeneous disease state, and GC can improve risk stratification to help personalize shared decision making.
Trial registration: ClinicalTrials.gov NCT00767286 NCT00769548 NCT00004054.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Drs
Figures

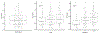

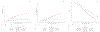
Comment in
-
Passing the Kool-Aid Point: mRNA Expression-Based Risk Classifiers in Localized Prostate Cancer Treatment Decision Making.Int J Radiat Oncol Biol Phys. 2023 Jul 1;116(3):530-532. doi: 10.1016/j.ijrobp.2022.12.002. Int J Radiat Oncol Biol Phys. 2023. PMID: 37270247 No abstract available.
References
-
- Muralidhar V, Zhang J, Wang Q, et al. Genomic Validation of 3-Tiered Clinical Subclassification of High-Risk Prostate Cancer. Int J Radiat Oncol Biol Phys 2019;105:621–7. - PubMed
-
- Jairath NK, Dal Pra A, Vince R Jr., et al. A Systematic Review of the Evidence for the Decipher Genomic Classifier in Prostate Cancer. Eur Urol 2021;79:374–83. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

