Metabolic regulation of the proteasome under hypoxia by Poldip2 controls fibrotic signaling in vascular smooth muscle cells
- PMID: 36596387
- PMCID: PMC10268434
- DOI: 10.1016/j.freeradbiomed.2022.12.098
Metabolic regulation of the proteasome under hypoxia by Poldip2 controls fibrotic signaling in vascular smooth muscle cells
Abstract
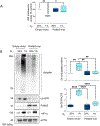
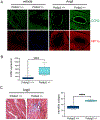
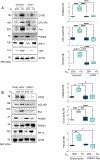
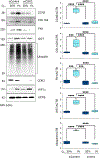
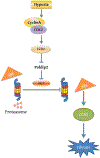
The polymerase delta interacting protein 2 (Poldip2) is a nuclear-encoded mitochondrial protein required for oxidative metabolism. Under hypoxia, Poldip2 expression is repressed by an unknown mechanism. Therefore, low levels of Poldip2 are required to maintain glycolytic metabolism. The Cellular Communication Network Factor 2 (CCN2, Connective tissue growth factor, CTGF) is a profibrogenic molecule highly expressed in cancer and vascular inflammation in advanced atherosclerosis. Because CCN2 is upregulated under hypoxia and is associated with glycolytic metabolism, we hypothesize that Poldip2 downregulation is responsible for the upregulation of profibrotic signaling under hypoxia. Here, we report that Poldip2 is repressed under hypoxia by a mechanism that requires the activation of the enhancer of zeste homolog 2 repressive complex (EZH2) downstream from the Cyclin-Dependent Kinase 2 (CDK2). Importantly, we found that Poldip2 repression is required for CCN2 expression downstream of metabolic inhibition of the ubiquitin-proteasome system (UPS)-dependent stabilization of the serum response factor. Pharmacological or gene expression inhibition of CDK2 under hypoxia reverses Poldip2 downregulation, the inhibition of the UPS, and the expression of CCN2, collagen, and fibronectin. Thus, our findings connect cell cycle regulation and proteasome activity to mitochondrial function and fibrotic responses under hypoxia.
Keywords: CCN2; Cell cycle; Hypoxia; Mitochondria; Poldip2; Proteasome.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures









Similar articles
-
Mitochondrial Protein Poldip2 (Polymerase Delta Interacting Protein 2) Controls Vascular Smooth Muscle Differentiated Phenotype by O-Linked GlcNAc (N-Acetylglucosamine) Transferase-Dependent Inhibition of a Ubiquitin Proteasome System.Circ Res. 2020 Jan 3;126(1):41-56. doi: 10.1161/CIRCRESAHA.119.315932. Epub 2019 Oct 28. Circ Res. 2020. PMID: 31656131 Free PMC article.
-
Polymerase delta-interacting protein 2 regulates collagen accumulation via activation of the Akt/mTOR pathway in vascular smooth muscle cells.J Mol Cell Cardiol. 2016 Mar;92:21-9. doi: 10.1016/j.yjmcc.2016.01.016. Epub 2016 Jan 19. J Mol Cell Cardiol. 2016. PMID: 26801741 Free PMC article.
-
Poldip2 is an oxygen-sensitive protein that controls PDH and αKGDH lipoylation and activation to support metabolic adaptation in hypoxia and cancer.Proc Natl Acad Sci U S A. 2018 Feb 20;115(8):1789-1794. doi: 10.1073/pnas.1720693115. Epub 2018 Feb 6. Proc Natl Acad Sci U S A. 2018. PMID: 29434038 Free PMC article.
-
Molecular regulation of CCN2 in the intervertebral disc: lessons learned from other connective tissues.Matrix Biol. 2013 Aug 8;32(6):298-306. doi: 10.1016/j.matbio.2013.03.006. Epub 2013 Apr 6. Matrix Biol. 2013. PMID: 23567513 Free PMC article. Review.
-
The Cytoskeletal Network Regulates Expression of the Profibrotic Genes PAI-1 and CTGF in Vascular Smooth Muscle Cells.Adv Pharmacol. 2018;81:79-94. doi: 10.1016/bs.apha.2017.08.006. Epub 2017 Oct 31. Adv Pharmacol. 2018. PMID: 29310804 Review.
Cited by
-
Combined inhibition of EZH2 and CDK4/6 perturbs endoplasmic reticulum-mitochondrial homeostasis and increases antitumor activity against glioblastoma.NPJ Precis Oncol. 2024 Jul 25;8(1):156. doi: 10.1038/s41698-024-00653-3. NPJ Precis Oncol. 2024. PMID: 39054369 Free PMC article.
-
Connective Tissue Growth Factor: Regulation, Diseases, and Drug Discovery.Int J Mol Sci. 2024 Apr 25;25(9):4692. doi: 10.3390/ijms25094692. Int J Mol Sci. 2024. PMID: 38731911 Free PMC article. Review.
-
Role of EZH2-mediated epigenetic modification on vascular smooth muscle in cardiovascular diseases: A mini-review.Front Pharmacol. 2024 Jun 27;15:1416992. doi: 10.3389/fphar.2024.1416992. eCollection 2024. Front Pharmacol. 2024. PMID: 38994197 Free PMC article. Review.
References
-
- Barman SA, Li X, Haigh S, Kondrikov D, Mahboubi K, Bordan Z, Stepp DW, Zhou J, Wang Y, Weintraub DS, Traber P, Snider W, Jonigk D, Sullivan J, Crislip GR, Butcher JT, Thompson J, Su Y, Chen F, Fulton DJR, Galectin-3 is expressed in vascular smooth muscle cells and promotes pulmonary hypertension through changes in proliferation, apoptosis, and fibrosis, Am J Physiol Lung Cell Mol Physiol 316(5) (2019) L784–L797. - PMC - PubMed
-
- Wiafe B, Adesida A, Churchill T, Adewuyi EE, Li Z, Metcalfe P, Hypoxia-increased expression of genes involved in inflammation, dedifferentiation, pro-fibrosis, and extracellular matrix remodeling of human bladder smooth muscle cells, In Vitro Cell Dev Biol Anim 53(1) (2017) 58–66. - PubMed
-
- Imanishi M, Tomita S, Ishizawa K, Kihira Y, Ueno M, Izawa-Ishizawa Y, Ikeda Y, Yamano N, Tsuchiya K, Tamaki T, Smooth muscle cell-specific Hif-1alpha deficiency suppresses angiotensin II-induced vascular remodelling in mice, Cardiovasc Res 102(3) (2014) 460–8. - PubMed
-
- Paredes F, Sheldon K, Lassegue B, Williams HC, Faidley EA, Benavides GA, Torres G, Sanhueza-Olivares F, Yeligar SM, Griendling KK, Darley-Usmar V, San Martin A, Poldip2 is an oxygen-sensitive protein that controls PDH and alphaKGDH lipoylation and activation to support metabolic adaptation in hypoxia and cancer, Proc Natl Acad Sci U S A 115(8) (2018) 1789–1794. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

