Circovirus Hepatitis Infection in Heart-Lung Transplant Patient, France
- PMID: 36596569
- PMCID: PMC9881760
- DOI: 10.3201/eid2902.221468
Circovirus Hepatitis Infection in Heart-Lung Transplant Patient, France
Abstract
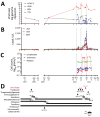
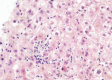
In March 2022, a 61-year-old woman in France who had received a heart-lung transplant sought treatment with chronic hepatitis mainly characterized by increased liver enzymes. After ruling out common etiologies, we used metagenomic next-generation sequencing to analyze a liver biopsy sample and identified an unknown species of circovirus, tentatively named human circovirus 1 (HCirV-1). We found no other viral or bacterial sequences. HCirV-1 shared 70% amino acid identity with the closest known viral sequences. The viral genome was undetectable in blood samples from 2017-2019, then became detectable at low levels in September 2020 and peaked at very high titers (1010 genome copies/mL) in January 2022. In March 2022, we found >108 genome copies/g or mL in the liver and blood, concomitant with hepatic cytolysis. We detected HCirV-1 transcripts in 2% of hepatocytes, demonstrating viral replication and supporting the role of HCirV-1 in liver damage.
Keywords: France; circoviruses; hepatitis; mNGS; metagenomic next-generation sequencing; solid organ transplants; viruses; zoonoses.
Figures


References
-
- World Health Organization. Acute hepatitis of unknown aetiology—the United Kingdom of Great Britain and Northern Ireland [cited 2022 Dec 15]. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON368
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

