Systematic review and individual-patient-data meta-analysis of non-invasive fibrosis markers for chronic hepatitis B in Africa
- PMID: 36596805
- PMCID: PMC9810658
- DOI: 10.1038/s41467-022-35729-w
Systematic review and individual-patient-data meta-analysis of non-invasive fibrosis markers for chronic hepatitis B in Africa
Abstract
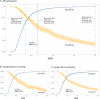
In sub-Saharan Africa, simple biomarkers of liver fibrosis are needed to scale-up hepatitis B treatment. We conducted an individual participant data meta-analysis of 3,548 chronic hepatitis B patients living in eight sub-Saharan African countries to assess the World Health Organization-recommended aspartate aminotransferase-to-platelet ratio index and two other fibrosis biomarkers using a Bayesian bivariate model. Transient elastography was used as a reference test with liver stiffness measurement thresholds at 7.9 and 12.2kPa indicating significant fibrosis and cirrhosis, respectively. At the World Health Organization-recommended cirrhosis threshold (>2.0), aspartate aminotransferase-to-platelet ratio index had sensitivity (95% credible interval) of only 16.5% (12.5-20.5). We identified an optimised aspartate aminotransferase-to-platelet ratio index rule-in threshold (>0.65) for liver stiffness measurement >12.2kPa with sensitivity and specificity of 56.2% (50.5-62.2) and 90.0% (89.0-91.0), and an optimised rule-out threshold (<0.36) with sensitivity and specificity of 80.6% (76.1-85.1) and 64.3% (62.8-65.8). Here we show that the World Health Organization-recommended aspartate aminotransferase-to-platelet ratio index threshold is inappropriately high in sub-Saharan Africa; improved rule-in and rule-out thresholds can optimise treatment recommendations in this setting.
© 2023. The Author(s).
Conflict of interest statement
The authors declare the following competing interests: M.L. and Y.S. have received consultancy fees and research funding from Gilead Sciences, USA. The other authors declare no competing interests.
Figures