Type I interferon signaling facilitates resolution of acute liver injury by priming macrophage polarization
- PMID: 36596875
- PMCID: PMC9886918
- DOI: 10.1038/s41423-022-00966-y
Type I interferon signaling facilitates resolution of acute liver injury by priming macrophage polarization
Abstract
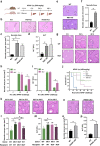
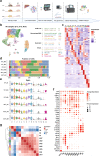
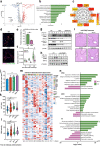
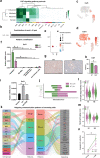
Due to their broad functional plasticity, myeloid cells contribute to both liver injury and recovery during acetaminophen overdose-induced acute liver injury (APAP-ALI). A comprehensive understanding of cellular diversity and intercellular crosstalk is essential to elucidate the mechanisms and to develop therapeutic strategies for APAP-ALI treatment. Here, we identified the function of IFN-I in the myeloid compartment during APAP-ALI. Utilizing single-cell RNA sequencing, we characterized the cellular atlas and dynamic progression of liver CD11b+ cells post APAP-ALI in WT and STAT2 T403A mice, which was further validated by immunofluorescence staining, bulk RNA-seq, and functional experiments in vitro and in vivo. We identified IFN-I-dependent transcriptional programs in a three-way communication pathway that involved IFN-I synthesis in intermediate restorative macrophages, leading to CSF-1 production in aging neutrophils that ultimately enabled Trem2+ restorative macrophage maturation, contributing to efficient liver repair. Overall, we uncovered the heterogeneity of hepatic myeloid cells in APAP-ALI at single-cell resolution and the therapeutic potential of IFN-I in the treatment of APAP-ALI.
Keywords: APAP-ALI; CSF1+ neutrophil; IFN-I; Macrophage polarization; STAT2 T403 phosphorylation; scRNA-seq.
© 2022. The Author(s), under exclusive licence to CSI and USTC.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

