Testing the effects of mass drug administration of azithromycin on mortality and other outcomes among 1-11-month-old infants in Mali (LAKANA): study protocol for a cluster-randomized, placebo-controlled, double-blinded, parallel-group, three-arm clinical trial
- PMID: 36597115
- PMCID: PMC9809521
- DOI: 10.1186/s13063-022-06966-7
Testing the effects of mass drug administration of azithromycin on mortality and other outcomes among 1-11-month-old infants in Mali (LAKANA): study protocol for a cluster-randomized, placebo-controlled, double-blinded, parallel-group, three-arm clinical trial
Abstract
Background: Mass drug administration (MDA) of azithromycin (AZI) has been shown to reduce under-5 mortality in some but not all sub-Saharan African settings. A large-scale cluster-randomized trial conducted in Malawi, Niger, and Tanzania suggested that the effect differs by country, may be stronger in infants, and may be concentrated within the first 3 months after treatment. Another study found no effect when azithromycin was given concomitantly with seasonal malaria chemoprevention (SMC). Given the observed heterogeneity and possible effect modification by other co-interventions, further trials are needed to determine the efficacy in additional settings and to determine the most effective treatment regimen.
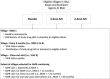
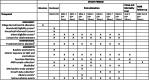
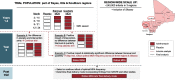
Methods: LAKANA stands for Large-scale Assessment of the Key health-promoting Activities of two New mass drug administration regimens with Azithromycin. The LAKANA trial is designed to address the mortality and health impacts of 4 or 2 annual rounds of azithromycin MDA delivered to 1-11-month-old (29-364 days) infants, in a high-mortality and malaria holoendemic Malian setting where there is a national SMC program. Participating villages (clusters) are randomly allocated in a ratio of 3:2:4 to three groups: placebo (control):4-dose AZI:2-dose AZI. The primary outcome measured is mortality. Antimicrobial resistance (AMR) will be monitored closely before, during, and after the intervention and both among those receiving and those not receiving MDA with the study drugs. Other outcomes, from a subset of villages, comprise efficacy outcomes related to morbidity, growth and nutritional status, outcomes related to the mechanism of azithromycin activity through measures of malaria parasitemia and inflammation, safety outcomes (AMR, adverse and serious adverse events), and outcomes related to the implementation of the intervention documenting feasibility, acceptability, and economic aspects. The enrolment commenced in October 2020 and is planned to be completed by the end of 2022. The expected date of study completion is December 2024.
Discussion: If LAKANA provides evidence in support of a positive mortality benefit resulting from azithromycin MDA, it will significantly contribute to the options for successfully promoting child survival in Mali, and elsewhere in sub-Saharan Africa.
Trial registration: ClinicalTrials.gov NCT04424511. Registered on 11 June 2020.
Keywords: Antibiotic; Antimicrobial resistance; Azithromycin; Growth; Infant; Infection; Inflammation; Morbidity; Mortality; Placebo; Randomized controlled trial.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- UN Inter-agency Group for Child Mortality Estimation . Levels & trends in child mortality: report 2021. 2021.
-
- UN General Assembly . Transforming our world: the 2030 agenda for sustainable development. 2015.
-
- Perin J, Mulick A, Yeung D, Villavicencio F, Lopez G, Strong KL, et al. Global, regional, and national causes of under-5 mortality in 2000–19: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet Child Adolesc Health. 2022;6(2):106–115. doi: 10.1016/S2352-4642(21)00311-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

