Differential vulnerability of the dentate gyrus to tauopathies in dementias
- PMID: 36597124
- PMCID: PMC9811688
- DOI: 10.1186/s40478-022-01485-7
Differential vulnerability of the dentate gyrus to tauopathies in dementias
Abstract
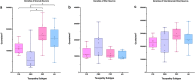
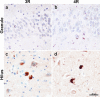
The dentate gyrus (DG), a key hippocampal subregion in memory processing, generally resists phosphorylated tau accumulation in the amnestic dementia of the Alzheimer's type due to Alzheimer's disease (DAT-AD), but less is known about the susceptibility of the DG to other tauopathies. Here, we report stereologic densities of total DG neurons and tau inclusions in thirty-two brains of human participants with autopsy-confirmed tauopathies with distinct isoform profiles-3R Pick's disease (PiD, N = 8), 4R corticobasal degeneration (CBD, N = 8), 4R progressive supranuclear palsy (PSP, N = 8), and 3/4R AD (N = 8). All participants were diagnosed during life with primary progressive aphasia (PPA), an aphasic clinical dementia syndrome characterized by progressive deterioration of language abilities with spared non-language cognitive abilities in early stages, except for five patients with DAT-AD as a comparison group. 51% of total participants were female. All specimens were stained immunohistochemically with AT8 to visualize tau pathology, and PPA cases were stained for Nissl substance to visualize neurons. Unbiased stereological analysis was performed in granule and hilar DG cells, and inclusion-to-neuron ratios were calculated. In the PPA group, PiD had highest mean total (granule + hilar) densities of DG tau pathology (p < 0.001), followed by CBD, AD, then PSP. PPA-AD cases showed more inclusions in hilar cells compared to granule cells, while the opposite was true in PiD and CBD. Inclusion-to-neuron ratios revealed, on average, 33% of all DG neurons in PiD cases contained a tau inclusion, compared to ~ 7% in CBD, 2% in AD, and 0.4% in PSP. There was no significant difference between DAT-AD and PPA-AD pathologic tau burden, suggesting that differences in DG burden are not specific to clinical phenotype. We conclude that the DG is differentially vulnerable to pathologic tau accumulation, raising intriguing questions about the structural integrity and functional significance of hippocampal circuits in neurodegenerative dementias.
Keywords: Dentate gyrus; Frontotemporal lobar degeneration; Primary progressive aphasia; Tauopathy.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

