Shedding light on structure, function and regulation of human sirtuins: a comprehensive review
- PMID: 36597461
- PMCID: PMC9805487
- DOI: 10.1007/s13205-022-03455-1
Shedding light on structure, function and regulation of human sirtuins: a comprehensive review
Abstract
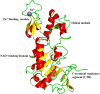
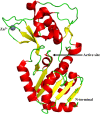
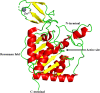
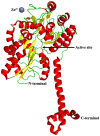
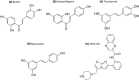
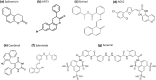
Sirtuins play an important role in signalling pathways associated with various metabolic regulations. They possess mono-ADP-ribosyltransferase or deacylase activity like demalonylase, deacetylase, depalmitoylase, demyristoylase and desuccinylase activity. Sirtuins are histone deacetylases which depends upon nicotinamide adenine dinucleotide (NAD) that deacetylate lysine residues. There are a total of seven human sirtuins that have been identified namely, SIRT1, SIRT2, SIRT3, SIRT4, SIRT5, SIRT6 and SIRT7. The subcellular location of mammalian sirtuins, SIRT1, SIRT6, and SIRT7 are in the nucleus; SIRT3, SIRT4, and SIRT5 are in mitochondria, and SIRT2 is in cytoplasm. Structurally sirtuins contains a N-terminal, a C-terminal and a Zn+ binding domain. The sirtuin family has been found to be crucial for maintaining lipid and glucose homeostasis, and also for regulating insulin secretion and sensitivity, DNA repair pathways, neurogenesis, inflammation, and ageing. Based on the literature, sirtuins are overexpressed and play an important role in tumorigenicity in various types of cancer such as non-small cell lung cancer, colorectal cancer, etc. In this review, we have discussed about the different types of human sirtuins along with their structural and functional features. We have also discussed about the various natural and synthetic regulators of sirtuin activities like resveratrol. Our overall study shows that the correct regulation of sirtuins can be a good target for preventing and treating various diseases for improving the human lifespan. To investigate the true therapeutic potential of sirtuin proteins and their efficacy in a variety of pathological diseases, a better knowledge of the link between the structure and function of sirtuin proteins would be necessary.
Keywords: Ageing; Deacetylase; Nicotinamide adenine dinucleotide; Rossmann fold; Sirtuins.
© King Abdulaziz City for Science and Technology 2022, Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Conflict of interestThe authors declare that they have no conflict of interest in the publication.
Figures


References
-
- Barber MF, Michishita-Kioi E, Xi Y, Tasselli L, Kioi M, Moqtaderi Z, Tennen RI, Paredes S, Young NL, Chen K, Struhl K, Garcia BA, Gozani O, Li W, Chua KF. SIRT7 links H3K18 deacetylation to maintenance of oncogenic transformation. Nature. 2012;487(7405):114–118. doi: 10.1038/nature11043. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

