Label-free impedimetric immunosensor for point-of-care detection of COVID-19 antibodies
- PMID: 36597510
- PMCID: PMC9805445
- DOI: 10.1038/s41378-022-00460-5
Label-free impedimetric immunosensor for point-of-care detection of COVID-19 antibodies
Abstract
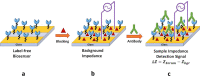
The COVID-19 pandemic has posed enormous challenges for existing diagnostic tools to detect and monitor pathogens. Therefore, there is a need to develop point-of-care (POC) devices to perform fast, accurate, and accessible diagnostic methods to detect infections and monitor immune responses. Devices most amenable to miniaturization and suitable for POC applications are biosensors based on electrochemical detection. We have developed an impedimetric immunosensor based on an interdigitated microelectrode array (IMA) to detect and monitor SARS-CoV-2 antibodies in human serum. Conjugation chemistry was applied to functionalize and covalently immobilize the spike protein (S-protein) of SARS-CoV-2 on the surface of the IMA to serve as the recognition layer and specifically bind anti-spike antibodies. Antibodies bound to the S-proteins in the recognition layer result in an increase in capacitance and a consequent change in the impedance of the system. The impedimetric immunosensor is label-free and uses non-Faradaic impedance with low nonperturbing AC voltage for detection. The sensitivity of a capacitive immunosensor can be enhanced by simply tuning the ionic strength of the sample solution. The device exhibits an LOD of 0.4 BAU/ml, as determined from the standard curve using WHO IS for anti-SARS-CoV-2 immunoglobulins; this LOD is similar to the corresponding LODs reported for all validated and established commercial assays, which range from 0.41 to 4.81 BAU/ml. The proof-of-concept biosensor has been demonstrated to detect anti-spike antibodies in sera from patients infected with COVID-19 within 1 h. Photolithographically microfabricated interdigitated microelectrode array sensor chips & label-free impedimetric detection of COVID-19 antibody.
Keywords: Biosensors; NEMS.
© The Author(s) 2023.
Conflict of interest statement
Conflict of interestThe authors declare no competing interests.
Figures
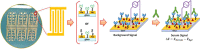
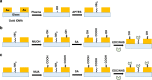
 ).
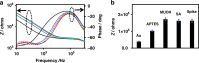
).
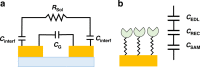
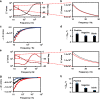
 ) COVID-19 positive serum, (
) COVID-19 positive serum, ( ) COVID-19 negative serum, (
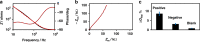
) COVID-19 negative serum, ( ) 1% milk blank samples, solid lines are drawn to guide the eyes, and (d, h) plot of the detection signal ΔZRel % (ΔZRel % = ΔZ/Zbgr × 100).Plots (a–d) and (e–h) correspond to EIS measurements made in 1× PBS or 0.001× PBS solution at pH 6.5, respectively.
) 1% milk blank samples, solid lines are drawn to guide the eyes, and (d, h) plot of the detection signal ΔZRel % (ΔZRel % = ΔZ/Zbgr × 100).Plots (a–d) and (e–h) correspond to EIS measurements made in 1× PBS or 0.001× PBS solution at pH 6.5, respectively.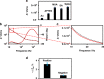


References
-
- Ducharme, J. World Health Organization Declares COVID-19 a “Pandemic”. Here’s What That Means, time.com. https://time.com/5791661/who-coronavirus-pandemic-declaration/(2020).
LinkOut - more resources
Full Text Sources
Miscellaneous
