This is a preprint.
Outpatient treatment of Covid-19 with metformin, ivermectin, and fluvoxamine and the development of Long Covid over 10-month follow-up
- PMID: 36597543
- PMCID: PMC9810227
- DOI: 10.1101/2022.12.21.22283753
Outpatient treatment of Covid-19 with metformin, ivermectin, and fluvoxamine and the development of Long Covid over 10-month follow-up
Abstract
Background: Long Covid is an emerging chronic illness potentially affecting millions, sometimes preventing the ability to work or participate in normal daily activities. COVID-OUT was an investigator-initiated, multi-site, phase 3, randomized, quadruple-blinded placebo-controlled clinical trial (NCT04510194). The design simultaneously assessed three oral medications (metformin, ivermectin, fluvoxamine) using two by three parallel treatment factorial assignment to efficiently share placebo controls and assessed Long Covid outcomes for 10 months to understand whether early outpatient treatment of SARS-CoV-2 with metformin, ivermectin, or fluvoxamine prevents Long Covid.
Methods: This was a decentralized, remotely delivered trial in the US of 1,125 adults age 30 to 85 with overweight or obesity, fewer than 7 days of symptoms, and enrolled within three days of a documented SARS-CoV-2 infection. Immediate release metformin titrated over 6 days to 1,500mg per day 14 days total; ivermectin 430mcg/kg/day for 3 days; fluvoxamine, 50mg on day one then 50mg twice daily through 14 days. Medical-provider diagnosis of Long Covid, reported by participant by day 300 after randomization was a pre-specified secondary outcome; the primary outcome of the trial was severe Covid by day 14.
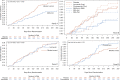
Result: The median age was 45 years (IQR 37 to 54), 56% female of whom 7% were pregnant. Two percent identified as Native American; 3.7% as Asian; 7.4% as Black/African American; 82.8% as white; and 12.7% as Hispanic/Latino. The median BMI was 29.8 kg/m2 (IQR 27 to 34); 51% had a BMI >30kg/m2. Overall, 8.4% reported having received a diagnosis of Long Covid from a medical provider: 6.3% in the metformin group and 10.6% in the metformin control; 8.0% in the ivermectin group and 8.1% in the ivermectin control; and 10.1% in the fluvoxamine group and 7.5% in the fluvoxamine control. The Hazard Ratio (HR) for Long Covid in the metformin group versus control was 0.58 (95% CI 0.38 to 0.88); 0.99 (95% CI 0.592 to 1.643) in the ivermectin group; and 1.36 in the fluvoxamine group (95% CI 0.785 to 2.385).
Conclusions: There was a 42% relative decrease in the incidence of Long Covid in the metformin group compared to its blinded control in a secondary outcome of this randomized phase 3 trial.
Figures


Similar articles
-
Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): a multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial.Lancet Infect Dis. 2023 Oct;23(10):1119-1129. doi: 10.1016/S1473-3099(23)00299-2. Epub 2023 Jun 8. Lancet Infect Dis. 2023. PMID: 37302406 Free PMC article. Clinical Trial.
-
Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for Covid-19.N Engl J Med. 2022 Aug 18;387(7):599-610. doi: 10.1056/NEJMoa2201662. N Engl J Med. 2022. PMID: 36070710 Free PMC article. Clinical Trial.
-
Favorable Antiviral Effect of Metformin on SARS-CoV-2 Viral Load in a Randomized, Placebo-Controlled Clinical Trial of COVID-19.Clin Infect Dis. 2024 Aug 16;79(2):354-363. doi: 10.1093/cid/ciae159. Clin Infect Dis. 2024. PMID: 38690892 Free PMC article. Clinical Trial.
-
Ivermectin for preventing and treating COVID-19.Cochrane Database Syst Rev. 2021 Jul 28;7(7):CD015017. doi: 10.1002/14651858.CD015017.pub2. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 Jun 21;6:CD015017. doi: 10.1002/14651858.CD015017.pub3. PMID: 34318930 Free PMC article. Updated.
-
Repaglinide : a pharmacoeconomic review of its use in type 2 diabetes mellitus.Pharmacoeconomics. 2004;22(6):389-411. doi: 10.2165/00019053-200422060-00005. Pharmacoeconomics. 2004. PMID: 15099124 Review.
Cited by
-
Association between accelerometer-measured irregular sleep duration and longitudinal changes in body mass index in older adults.Int J Obes (Lond). 2025 Jul;49(7):1280-1289. doi: 10.1038/s41366-025-01768-8. Epub 2025 Apr 6. Int J Obes (Lond). 2025. PMID: 40189712
References
-
- CDC. Post-Covid Conditions: Information for Healthcare Providers. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-c.... Published 2022. Accessed 11 Dec, 2022.
-
- Cutler DM. The Costs of Long COVID. JAMA Health Forum. 2022;3(5):e221809. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
