Circulating Spike Protein Detected in Post-COVID-19 mRNA Vaccine Myocarditis
- PMID: 36597886
- PMCID: PMC10010667
- DOI: 10.1161/CIRCULATIONAHA.122.061025
Circulating Spike Protein Detected in Post-COVID-19 mRNA Vaccine Myocarditis
Abstract
Background: Cases of adolescents and young adults developing myocarditis after vaccination with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-targeted mRNA vaccines have been reported globally, but the underlying immunoprofiles of these individuals have not been described in detail.
Methods: From January 2021 through February 2022, we prospectively collected blood from 16 patients who were hospitalized at Massachusetts General for Children or Boston Children's Hospital for myocarditis, presenting with chest pain with elevated cardiac troponin T after SARS-CoV-2 vaccination. We performed extensive antibody profiling, including tests for SARS-CoV-2-specific humoral responses and assessment for autoantibodies or antibodies against the human-relevant virome, SARS-CoV-2-specific T-cell analysis, and cytokine and SARS-CoV-2 antigen profiling. Results were compared with those from 45 healthy, asymptomatic, age-matched vaccinated control subjects.
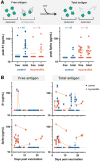
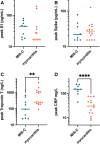
Results: Extensive antibody profiling and T-cell responses in the individuals who developed postvaccine myocarditis were essentially indistinguishable from those of vaccinated control subjects, despite a modest increase in cytokine production. A notable finding was that markedly elevated levels of full-length spike protein (33.9±22.4 pg/mL), unbound by antibodies, were detected in the plasma of individuals with postvaccine myocarditis, whereas no free spike was detected in asymptomatic vaccinated control subjects (unpaired t test; P<0.0001).
Conclusions: Immunoprofiling of vaccinated adolescents and young adults revealed that the mRNA vaccine-induced immune responses did not differ between individuals who developed myocarditis and individuals who did not. However, free spike antigen was detected in the blood of adolescents and young adults who developed post-mRNA vaccine myocarditis, advancing insight into its potential underlying cause.
Keywords: COVID-19; SARS-CoV-2; mRNA; myocarditis; vaccine.
Figures


Comment in
-
Response by Yonker et al to Letter Regarding Article, "Circulating Spike Protein Detected in Post-COVID-19 mRNA Vaccine Myocarditis".Circulation. 2023 Sep 12;148(11):910-911. doi: 10.1161/CIRCULATIONAHA.123.065629. Epub 2023 Sep 11. Circulation. 2023. PMID: 37695829 No abstract available.
-
Letter by Schwartz and Prasad Regarding Article, "Circulating Spike Protein Detected in Post-COVID-19 mRNA Vaccine Myocarditis".Circulation. 2023 Sep 12;148(11):908-909. doi: 10.1161/CIRCULATIONAHA.123.064414. Epub 2023 Sep 11. Circulation. 2023. PMID: 37695830 No abstract available.
-
Letter by Cosentino and Marino Regarding Article, "Circulating Spike Protein Detected in Post-COVID-19 mRNA Vaccine Myocarditis".Circulation. 2023 Sep 12;148(11):906-907. doi: 10.1161/CIRCULATIONAHA.123.064000. Epub 2023 Sep 11. Circulation. 2023. PMID: 37695833 No abstract available.
References
-
- Walter EB, Talaat KR, Sabharwal C, Gurtman A, Lockhart S, Paulsen GC, Barnett ED, Munoz FM, Maldonado Y, Pahud BA, et al. ; C4591001 Clinical Trial Group. Evaluation of the BNT162b2 Covid-19 vaccine in children 5 to 11 years of age. N Engl J Med. 2022;386:35–46. doi: 10.1056/NEJMoa2116298 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

