Th2 high and mast cell gene signatures are associated with corticosteroid sensitivity in COPD
- PMID: 36598042
- PMCID: PMC10086461
- DOI: 10.1136/thorax-2021-217736
Th2 high and mast cell gene signatures are associated with corticosteroid sensitivity in COPD
Abstract
Rationale: Severe asthma and chronic obstructive pulmonary disease (COPD) share common pathophysiological traits such as relative corticosteroid insensitivity. We recently published three transcriptome-associated clusters (TACs) using hierarchical analysis of the sputum transcriptome in asthmatics from the Unbiased Biomarkers for the Prediction of Respiratory Disease Outcomes (U-BIOPRED) cohort comprising one Th2-high inflammatory signature (TAC1) and two Th2-low signatures (TAC2 and TAC3).
Objective: We examined whether gene expression signatures obtained in asthma can be used to identify the subgroup of patients with COPD with steroid sensitivity.
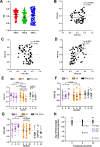
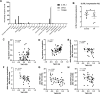
Methods: Using gene set variation analysis, we examined the distribution and enrichment scores (ES) of the 3 TACs in the transcriptome of bronchial biopsies from 46 patients who participated in the Groningen Leiden Universities Corticosteroids in Obstructive Lung Disease COPD study that received 30 months of treatment with inhaled corticosteroids (ICS) with and without an added long-acting β-agonist (LABA). The identified signatures were then associated with longitudinal clinical variables after treatment. Differential gene expression and cellular convolution were used to define key regulated genes and cell types.
Measurements and main results: Bronchial biopsies in patients with COPD at baseline showed a wide range of expression of the 3 TAC signatures. After ICS±LABA treatment, the ES of TAC1 was significantly reduced at 30 months, but those of TAC2 and TAC3 were unaffected. A corticosteroid-sensitive TAC1 signature was developed from the TAC1 ICS-responsive genes. This signature consisted of mast cell-specific genes identified by single-cell RNA-sequencing and positively correlated with bronchial biopsy mast cell numbers following ICS±LABA. Baseline levels of gene transcription correlated with the change in RV/TLC %predicted following 30-month ICS±LABA.
Conclusion: Sputum-derived transcriptomic signatures from an asthma cohort can be recapitulated in bronchial biopsies of patients with COPD and identified a signature of airway mast cells as a predictor of corticosteroid responsiveness.
Keywords: COPD pathology; airway epithelium; oxidative stress.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


Comment in
-
Inhaled corticosteroid responses in COPD: do mast cells hold the answer?Thorax. 2023 Apr;78(4):323-324. doi: 10.1136/thorax-2022-219534. Epub 2022 Dec 7. Thorax. 2023. PMID: 36598041 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
