Combined Immune Checkpoint Blockade Enhances Antiviral Immunity against Bovine Leukemia Virus
- PMID: 36598199
- PMCID: PMC9888214
- DOI: 10.1128/jvi.01430-22
Combined Immune Checkpoint Blockade Enhances Antiviral Immunity against Bovine Leukemia Virus
Abstract
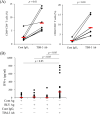
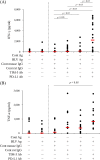
Bovine leukemia virus (BLV) is a retrovirus that causes enzootic bovine leukosis (EBL) in cattle and is widespread in many countries, including Japan. Recent studies have revealed that the expression of immunoinhibitory molecules, such as programmed death-1 (PD-1) and PD-ligand 1, plays a critical role in immunosuppression and disease progression during BLV infection. In addition, a preliminary study has suggested that another immunoinhibitory molecule, T-cell immunoglobulin domain and mucin domain-3 (TIM-3), is involved in immunosuppression during BLV infection. Therefore, this study was designed to further elucidate the immunoinhibitory role of immune checkpoint molecules in BLV infection. TIM-3 expression was upregulated on peripheral CD4+ and CD8+ T cells in BLV-infected cattle. Interestingly, in EBL cattle, CD4+ and CD8+ T cells infiltrating lymphomas expressed TIM-3. TIM-3 and PD-1 were upregulated and coexpressed in peripheral CD4+ and CD8+ T cells from BLV-infected cattle. Blockade by anti-bovine TIM-3 monoclonal antibody increased CD69 expression on T cells and gamma interferon (IFN-γ) production from peripheral blood mononuclear cells from BLV-infected cattle. A syncytium formation assay also demonstrated the antiviral effects of TIM-3 blockade against BLV infection. The combined inhibition of TIM-3 and PD-1 pathways significantly enhanced IFN-γ production and antiviral efficacy compared to inhibition alone. In conclusion, the combined blockade of TIM-3 and PD-1 pathways shows strong immune activation and antiviral effects and has potential as a novel therapeutic method for BLV infection. IMPORTANCE Enzootic bovine leukosis caused by bovine leukemia virus (BLV) is an important viral disease in cattle, causing severe economic losses to the cattle industry worldwide. The molecular mechanisms of BLV-host interactions are complex. Previously, it was found that immune checkpoint molecules, such as PD-1, suppress BLV-specific Th1 responses as the disease progresses. To date, most studies have focused only on how PD-1 facilitates escape from host immunity in BLV-infected cattle and the antiviral effects of the PD-1 blockade. In contrast, how T-cell immunoglobulin domain and mucin domain-3 (TIM-3), another immune checkpoint molecule, regulates anti-BLV immune responses is rarely reported. It is also unclear why PD-1 inhibition alone was insufficient to exert anti-BLV effects in previous clinical studies. In this study, the expression profile of TIM-3 in T cells derived from BLV-infected cattle suggested that TIM-3 upregulation is a cause of immunosuppression in infected cattle. Based on these results, anti-TIM-3 antibody was used to experimentally evaluate its function in influencing immunity against BLV. Results indicated that TIM-3 upregulation induced by BLV infection suppressed T-cell activation and antiviral cytokine production. Some T cells coexpressed PD-1 and TIM-3, indicating that simultaneous inhibition of PD-1 and TIM-3 with their respective antibodies synergistically restored antiviral immunity. This study could open new avenues for treating bovine chronic infections.
Keywords: PD-L1; TIM-3; bovine leukemia virus; cattle; lymphoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, Freeman GJ, Kuchroo VK. 2002. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 415:536–541. 10.1038/415536a. - DOI - PubMed
-
- Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM, Gyenes G, Vali B, Hyrcza MD, Yue FY, Kovacs C, Sassi A, Loutfy M, Halpenny R, Persad D, Spotts G, Hecht FM, Chun TW, McCune JM, Kaul R, Rini JM, Nixon DF, Ostrowski MA. 2008. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med 205:2763–2779. 10.1084/jem.20081398. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

