Efgartigimod improved health-related quality of life in generalized myasthenia gravis: results from a randomized, double-blind, placebo-controlled, phase 3 study (ADAPT)
- PMID: 36598575
- PMCID: PMC10025199
- DOI: 10.1007/s00415-022-11517-w
Efgartigimod improved health-related quality of life in generalized myasthenia gravis: results from a randomized, double-blind, placebo-controlled, phase 3 study (ADAPT)
Abstract
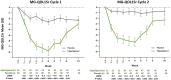
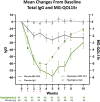
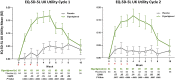
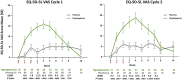
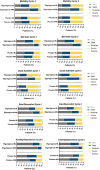
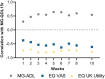
There are substantial disease and health-related quality-of-life (HRQoL) burdens for many patients with myasthenia gravis (MG), especially for those whose disease symptoms are not well controlled. HRQoL measures such as the Myasthenia Gravis Quality of Life 15-item revised (MG-QOL15r) and EuroQoL 5-Dimensions 5-Levels (EQ-5D-5L) are vital for evaluating the clinical benefit of therapeutic interventions in patients with MG, as they assess the burden of disease and the effectiveness of treatment, as perceived by patients. The phase 3 ADAPT study (NCT03669588) demonstrated that efgartigimod-a novel neonatal Fc receptor inhibitor-was well tolerated and that acetylcholine receptor antibody-positive (AChR-Ab+) participants who received efgartigimod had statistically significant improvements in MG-specific clinical scale scores. The ancillary data reported here, which cover an additional treatment cycle, show that these participants had similar significant improvements in HRQoL measures, the MG-QOL15r and EQ-5D-5L utility and visual analog scales, and that these improvements were maintained in the second treatment cycle. Positive effects on HRQoL were rapid, seen as early as the first week of treatment in both treatment cycles, and maintained for up to 4 weeks in the follow-up-only portion of treatment cycles. The pattern of improvements in HRQoL paralleled changes in immunoglobulin G level, and correlational analyses show that improvements were consistent across HRQoL measures and with clinical efficacy measures in the ADAPT study. The substantial and durable improvements in HRQoL end points in this study demonstrate the broader benefit of treatment with efgartigimod beyond relief of immediate signs and symptoms of gMG.
Keywords: Efgartigimod; Generalized myasthenia gravis; HRQoL; Patient-reported outcomes; Quality of life; gMG.
© 2023. The Author(s).
Conflict of interest statement
This study was sponsored by argenx SE, the manufacturer of efgartigimod alfa, which is approved for use in patients with AChR-Ab+ generalized myasthenia gravis by the US Food and Drug Administration. Sihui Zhao, PhD; Deborah Gelinas, MD; Cynthia Z. Qi; Silvia Chiroli, MD; and Glenn Phillips, PhD, are employees of argenx. Silvia Chiroli, MD, is a stock shareholder in addition to being an employee of argenx. Francesco Saccà, MD, has received honoraria for public speaking from Alexion, Biogen, Mylan, Novartis, Roche, Sanofi, and Teva; served on advisory boards for Alexion, Almirall, argenx, AveXis, Biogen, Forward Pharma, Lexeo Therapeutics, Merck, Novartis, Roche, Sanofi, and Takeda; and served as clinical trial principal investigator for Alexion, argenx, Novartis, Prilenia, and Sanofi. Carolina Barnett, MD, PhD, has received grant support from the US Department of Defense, US National Institutes of Health, Muscular Dystrophy Canada, MGNet, Grifols, and Octapharma. She has participated in advisory boards for Alexion, Sanofi, and argenx and been a consultant for CSL, Alexion, and argenx. Jan J.G.M. Verschuuren, MD, has received grant support from the Prinses Beatrix Spierfonds, Health Holland and participated in consultancies for argenx, Alexion, Ra Pharmaceuticals, and NMD Pharma. Reimbursements were received by the Leiden University Medical Centre. He is co-inventor on patent applications based on MuSK-related research. He is a member of the European Reference Network for Rare Neuromuscular Diseases. Stojan Peric, MD, PhD, served as site principal investigator for MG clinical trials sponsored by argenx, Ra/UCB, and Takeda/Millennium and is a consultant for argenx. Tuan Vu, MD, served as site principal investigator for MG clinical trials sponsored by Alexion, argenx, Ra/UCB, Horizon/Viela Bio, Janssen/Momenta, Regeneron, and Cartesian Therapeutics and is a consultant for UCB, Alexion, and argenx.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

