Light-induced changes of far-red excited chlorophyll fluorescence: further evidence for variable fluorescence of photosystem I in vivo
- PMID: 36598714
- PMCID: PMC9958156
- DOI: 10.1007/s11120-022-00994-9
Light-induced changes of far-red excited chlorophyll fluorescence: further evidence for variable fluorescence of photosystem I in vivo
Abstract
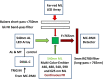
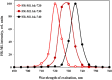
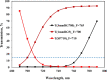
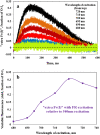
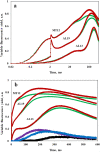
Recently, the long-standing paradigm of variable chlorophyll (Chl) fluorescence (Fv) in vivo originating exclusively from PSII was challenged, based on measurements with green algae and cyanobacteria (Schreiber and Klughammer 2021, PRES 149, 213-231). Fv(I) was identified by comparing light-induced changes of Fv > 700 nm and Fv < 710 nm. The Fv(I) induced by strong light was about 1.5 × larger in Fv > 700 nm compared to Fv < 710 nm. In the present communication, concentrating on the model green alga Chlorella vulgaris, this work is extended by comparing the light-induced changes of long-wavelength fluorescence (> 765 nm) that is excited by either far-red light (720 nm, mostly absorbed in PSI) or visible light (540 nm, absorbed by PSI and PSII). Polyphasic rise curves of Fv induced by saturating 540 nm light are measured, which after normalization of the initial O-I1 rises, assumed to reflect Fv(II), display a 2 × higher I2-P transient with 720 nm excitation (720ex) compared with 540ex. Analysis of the Fo(I) contributions to Fo(720ex) and Fo(540ex) reveals that also Fo(I)720ex is 2 × higher than Fo(I)540ex, which supports the notion that the whole I2-P transient is due to Fv(I). The twofold increase of the excitation ratio of F(I)/F(II) from 680 to 720 nm is much smaller than the eight-tenfold increase of PSI/PSII known from action spectra. It is suggested that the measured F > 765 nm is not representative for the bulk chlorophyll of PSI, but rather reflects a small fraction of far-red absorbing chlorophyll forms ("red Chls") with particular properties. Based on the same approach (comparison of polyphasic rise curves measured with 720ex and 540ex), the existence of Fv(I) is confirmed in a variety of other photosynthetic organisms (cyanobacteria, moss, fern, higher plant leaves).
Keywords: Chlorella; Far-red absorption; Far-red excited chlorophyll fluorescence; Fv(I); F > 765 nm; MULTI-COLOR-PAM; Polyphasic fluorescence rise O-I1-I2-P; Room temperature fluorescence emission; “Red Chls”.
© 2023. The Author(s).
Conflict of interest statement
There is no conflict of interest.
Figures








References
-
- Bell AJ, Frankel LK, Bricker TM. High yield non-detergent isolation of photosystem I-light-harvesting chlorophyll II membranes from spinach thylakoids: implications for the organization of the PS I antennae in higher plants. J Biol Chem. 2015;290:18429–18437. doi: 10.1074/jbc.M115.663872. - DOI - PMC - PubMed
-
- Butler WL. Energy distribution in the photochemical apparatus of photosynthesis. Ann Rev Plant Physiol. 1978;29:345–378. doi: 10.1146/annurev.pp.29.060178.002021. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

