Frailty and Effects of a Multidomain Physical Rehabilitation Intervention Among Older Patients Hospitalized for Acute Heart Failure: A Secondary Analysis of a Randomized Clinical Trial
- PMID: 36598761
- PMCID: PMC9857661
- DOI: 10.1001/jamacardio.2022.4903
Frailty and Effects of a Multidomain Physical Rehabilitation Intervention Among Older Patients Hospitalized for Acute Heart Failure: A Secondary Analysis of a Randomized Clinical Trial
Abstract
Importance: Frailty is common among older patients with acute decompensated heart failure (ADHF) and is associated with worse quality of life (QOL) and a higher risk of clinical events. Frailty can also limit recovery and response to interventions. In the Rehabilitation Therapy in Older Acute Heart Failure Patients (REHAB-HF) trial, a 3-month innovative, early, transitional, tailored, multidomain physical rehabilitation intervention improved physical function and QOL (vs usual care) in older patients with ADHF.
Objective: To evaluate whether baseline frailty modified the benefits of the physical rehabilitation intervention among patients with ADHF enrolled in the REHAB-HF trial and to assess the association between changes in frailty with the risk of adverse clinical outcomes on follow-up.
Design, setting, and participants: This prespecified secondary analysis of the REHAB-HF trial, a multicenter randomized clinical trial, included 337 patients 60 years and older hospitalized for ADHF. Patients were enrolled from September 17, 2014, through September 19, 2019. Participants were stratified across baseline frailty strata as assessed using modified Fried criteria. Data were analyzed from July 2021 to September 2022.
Interventions: Physical rehabilitation intervention or attention control.
Main outcomes and measures: Primary outcome was the Short Physical Performance Battery (SPPB) score at 3 months. Clinical outcomes included all-cause hospitalization or mortality at 6 months.
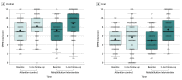
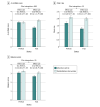
Results: This prespecified secondary analysis included 337 participants; 181 (53.7%) were female, 167 (49.6%) were Black, and the mean (SD) age was 72 (8) years. A total of 192 (57.0%) were frail and 145 (43.0%) were prefrail at baseline. A significant interaction was observed between baseline frailty status and the treatment arm for the primary trial end point of overall SPPB score, with a 2.6-fold larger improvement in SPPB with intervention among frail patients (2.1; 95% CI, 1.3-2.9) vs prefrail patients (0.8; 95% CI, -0.1 to 1.6; P for interaction = .03). Trends consistently favored a larger intervention effect size, with significant improvement among frail vs prefrail participants for 6-minute walk distance, QOL, and the geriatric depression score, but interactions did not achieve significance.
Conclusions and relevance: In this prespecified secondary analysis of the REHAB-HF trial, patients with ADHF with worse baseline frailty status had a more significant improvement in physical function in response to an innovative, early, transitional, tailored, multidomain physical rehabilitation intervention than those who were prefrail.
Trial registration: Clinical Trials.gov Identifier: NCT02196038.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

