Re-formation of synaptic connectivity in dissociated human stem cell-derived retinal organoid cultures
- PMID: 36598946
- PMCID: PMC9926218
- DOI: 10.1073/pnas.2213418120
Re-formation of synaptic connectivity in dissociated human stem cell-derived retinal organoid cultures
Abstract
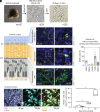
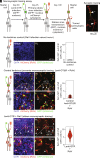
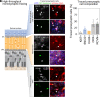
Human pluripotent stem cell (hPSC)-derived retinal organoids (ROs) can efficiently and reproducibly generate retinal neurons that have potential for use in cell replacement strategies [Capowski et al., Development 146, dev171686 (2019)]. The ability of these lab-grown retinal neurons to form new synaptic connections after dissociation from ROs is key to building confidence in their capacity to restore visual function. However, direct evidence of reestablishment of retinal neuron connectivity via synaptic tracing has not been reported to date. The present study employs an in vitro, rabies virus-based, monosynaptic retrograde tracing assay [Wickersham et al., Neuron 53, 639-647 (2007); Sun et al., Mol. Neurodegener. 14, 8 (2019)] to identify de novo synaptic connections among early retinal cell types following RO dissociation. A reproducible, high-throughput approach for labeling and quantifying traced retinal cell types was developed. Photoreceptors and retinal ganglion cells-the primary neurons of interest for retinal cell replacement-were the two major contributing populations among the traced presynaptic cells. This system provides a platform for assessing synaptic connections in cultured retinal neurons and sets the stage for future cell replacement studies aimed at characterizing or enhancing synaptogenesis. Used in this manner, in vitro synaptic tracing is envisioned to complement traditional preclinical animal model testing, which is limited by evolutionary incompatibilities in synaptic machinery inherent to human xenografts.
Keywords: human pluripotent stem cells; photoreceptors; retinal organoid; synapses; trans-synaptic tracing.
Conflict of interest statement
The authors declare competing interests and have patent filings to disclose. D.M.G. has declared intellectual rights for production of 3D ROs through the Wisconsin Alumni Research Foundation, Madison, WI (US PTO No. 9,328,328). The authors have organizational affiliations and research support to disclose. D.M.G. has an ownership interest in and receives grant support from Opsis Therapeutics LLC, which has licensed the technology to generate ROs from hPSCs utilized in this publication. The terms of this arrangement have been reviewed and approved by the University of Wisconsin-Madison in accordance with its conflict-of-interest policies. All other authors declare no competing interests.
Figures
References
-
- Cajal S. R. y., Estructura de los centros nerviosos de las aves. Revista Trimestral de Histología Normal y Patológica, 1, 1–10 (1888).
-
- Jones B. W., Marc R. E., Retinal remodeling during retinal degeneration. Exp. Eye Res. 81, 123–137 (2005). - PubMed
-
- Quigley H. A., Glaucoma. Lancet 377, 1367–1377 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

