A nanofiber-hydrogel composite improves tissue repair in a rat model of Crohn's disease perianal fistulas
- PMID: 36598982
- PMCID: PMC9812382
- DOI: 10.1126/sciadv.ade1067
A nanofiber-hydrogel composite improves tissue repair in a rat model of Crohn's disease perianal fistulas
Abstract
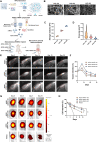
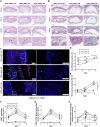
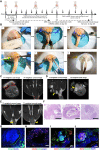
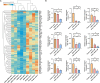
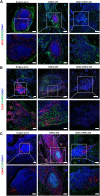
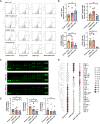
Perianal fistulas (PAFs) represent a severe complication of Crohn's disease (CD). Despite the advent of biologic and small-molecule therapeutics for luminal disease, PAFs in CD (CD-PAF) are relatively resistant to treatment, with less than 50% responding to any therapy. We report an injectable, biodegradable, mechanically fragmented nanofiber-hydrogel composite (mfNHC) loaded with adipose-derived stem cells (ADSCs) for the treatment of fistulas in a rat model of CD-PAF. The ADSC-loaded mfNHC results in a higher degree of healing when compared to surgical treatment of fistulas, which is a standard treatment. The volume of fistulas treated with mfNHC is decreased sixfold compared to the surgical treatment control. Molecular studies reveal that utilization of mfNHC reduced local inflammation and improved tissue regeneration. This study demonstrates that ADSC-loaded mfNHC is a promising therapy for CD-PAF, and warrants further studies to advance mfNHC toward clinical translation.
Figures

References
-
- M. L. Ganz, R. Sugarman, R. Wang, B. B. Hansen, J. Håkan-Bloch,The economic and health-related impact of Crohn’s disease in the United States: Evidence from a nationally representative survey. Inflamm. Bowel Dis. 22,1032–1041 (2016). - PubMed
-
- S. J. Bell, A. B. Williams, P. Wiesel, K. Wilkinson, R. C. Cohen, M. A. Kamm,The clinical course of fistulating Crohn’s disease. Aliment. Pharmacol. Ther. 17,1145–1151 (2003). - PubMed
-
- T. W. Eglinton, M. L. Barclay, R. B. Gearry, F. A. Frizelle,The spectrum of perianal Crohn’s disease in a population-based cohort. Dis. Colon Rectum 55,773–777 (2012). - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

