Assessment of systemic AAV-microdystrophin gene therapy in the GRMD model of Duchenne muscular dystrophy
- PMID: 36599002
- PMCID: PMC11107748
- DOI: 10.1126/scitranslmed.abo1815
Assessment of systemic AAV-microdystrophin gene therapy in the GRMD model of Duchenne muscular dystrophy
Abstract
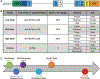
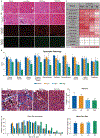
Duchenne muscular dystrophy (DMD) is a progressive muscle wasting disease caused by the absence of dystrophin, a membrane-stabilizing protein encoded by the DMD gene. Although mouse models of DMD provide insight into the potential of a corrective therapy, data from genetically homologous large animals, such as the dystrophin-deficient golden retriever muscular dystrophy (GRMD) model, may more readily translate to humans. To evaluate the clinical translatability of an adeno-associated virus serotype 9 vector (AAV9)-microdystrophin (μDys5) construct, we performed a blinded, placebo-controlled study in which 12 GRMD dogs were divided among four dose groups [control, 1 × 1013 vector genomes per kilogram (vg/kg), 1 × 1014 vg/kg, and 2 × 1014 vg/kg; n = 3 each], treated intravenously at 3 months of age with a canine codon-optimized microdystrophin construct, rAAV9-CK8e-c-μDys5, and followed for 90 days after dosing. All dogs received prednisone (1 milligram/kilogram) for a total of 5 weeks from day -7 through day 28. We observed dose-dependent increases in tissue vector genome copy numbers; μDys5 protein in multiple appendicular muscles, the diaphragm, and heart; limb and respiratory muscle functional improvement; and reduction of histopathologic lesions. As expected, given that a truncated dystrophin protein was generated, phenotypic test results and histopathologic lesions did not fully normalize. All administrations were well tolerated, and adverse events were not seen. These data suggest that systemically administered AAV-microdystrophin may be dosed safely and could provide therapeutic benefit for patients with DMD.
Figures
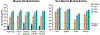



References
-
- Roberts M, Dickson G, The future of Duchenne muscular dystrophy gene therapy: shrinking the dystrophin gene. Curr. Opin. Mol. Ther. 4, 343–348 (2002). - PubMed
-
- Wells DJ, Ferrer A, Wells KE, Immunological hurdles in the path to gene therapy for Duchenne muscular dystrophy. Expert Rev. Mol. Med. 4, 1–23 (2002). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

