Overall Survival With Daratumumab, Lenalidomide, and Dexamethasone in Previously Treated Multiple Myeloma (POLLUX): A Randomized, Open-Label, Phase III Trial
- PMID: 36599114
- PMCID: PMC10022849
- DOI: 10.1200/JCO.22.00940
Overall Survival With Daratumumab, Lenalidomide, and Dexamethasone in Previously Treated Multiple Myeloma (POLLUX): A Randomized, Open-Label, Phase III Trial
Abstract
Purpose: With the initial analysis of POLLUX at a median follow-up of 13.5 months, daratumumab in combination with lenalidomide and dexamethasone (D-Rd) significantly prolonged progression-free survival versus lenalidomide and dexamethasone (Rd) alone in patients with relapsed or refractory multiple myeloma (RRMM). We report updated efficacy and safety results at the time of final analysis for overall survival (OS).
Methods: POLLUX was a multicenter, randomized, open-label, phase III study during which eligible patients with ≥ 1 line of prior therapy were randomly assigned 1:1 to D-Rd or Rd until disease progression or unacceptable toxicity. After positive primary analysis and protocol amendment, patients receiving Rd were offered daratumumab monotherapy after disease progression.
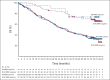
Results: Significant OS benefit was observed with D-Rd (hazard ratio, 0.73; 95% CI, 0.58 to 0.91; P = .0044) at a median (range) follow-up of 79.7 months (0.0-86.5). The median OS was 67.6 months for D-Rd compared with 51.8 months for Rd. Prespecified analyses demonstrated an improved OS with D-Rd versus Rd in most subgroups, including patients age ≥ 65 years and patients with one, two, or three prior lines of therapy, International Staging System stage III disease, high-risk cytogenetic abnormalities, and refractoriness to their last prior line of therapy or a proteasome inhibitor. The most common (≥ 10%) grade 3/4 treatment-emergent adverse events with D-Rd versus Rd were neutropenia (57.6% v 41.6%), anemia (19.8% v 22.4%), pneumonia (17.3% v 11.0%), thrombocytopenia (15.5% v 15.7%), and diarrhea (10.2% v 3.9%).
Conclusion: D-Rd significantly extended OS versus Rd alone in patients with RRMM. To our knowledge, for the first time, our findings, together with the OS benefit observed with daratumumab plus bortezomib and dexamethasone in the phase III CASTOR trial, demonstrate OS improvement with daratumumab-containing regimens in RRMM (ClinicalTrials.gov identifier: NCT02076009 [POLLUX]).
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures



References
-
- de Weers M, Tai YT, van der Veer MS, et al. : Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol 186:1840-1848, 2011 - PubMed
-
- Lammerts van Bueren J, Jakobs D, Kaldenhoven N, et al. : Direct in vitro comparison of daratumumab with surrogate analogs of CD38 antibodies MOR03087, SAR650984 and Ab79. Blood 124:3474, 2014
-
- Overdijk MB, Jansen JH, Nederend M, et al. : The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via Fcgamma receptor-mediated cross-linking. J Immunol 197:807-813, 2016 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

