Implications and prognostic impact of mass spectrometry in patients with newly-diagnosed multiple myeloma
- PMID: 36599831
- PMCID: PMC9812999
- DOI: 10.1038/s41408-022-00772-9
Implications and prognostic impact of mass spectrometry in patients with newly-diagnosed multiple myeloma
Abstract
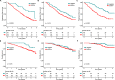
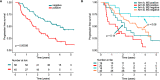
Mass spectrometry (MS) is a promising tool for monitoring monoclonal protein in plasma cell dyscrasias. We included 480 transplant-eligible newly-diagnosed multiple myeloma (MM) patients from the GMMG-MM5 trial (EudraCT No. 2010-019173-16) and performed a retrospective MS analysis at baseline (480 patients) and at the pre-defined, consecutive time points after induction (444 patients), prior to maintenance (305 patients) and after one year of maintenance (227 patients). We found that MS negativity was significantly associated with improved progression-free survival (PFS) even in patients with complete response (CR) at all investigated follow-up time points. The prognostic impact was independent of established risk factors, such as the revised International Staging System. Combining MS and baseline cytogenetics improved the prediction of outcome: MS-positive patients with high-risk cytogenetics had a dismal PFS of 1.9 years (95% confidence interval [CI]: 1.6-2.3 years) from the start of maintenance. Testing the value of sequential MS prior to and after one year of maintenance, patients converting from MS positivity to negativity had an excellent PFS (median not reached) while patients converting from MS negativity to positivity progressed early (median 0.6 years, 95% CI: 0.3-not reached). Among patients with sustained MS positivity, the baseline high-risk cytogenetic status had a significant impact and defined a group with poor PFS. Combining minimal residual disease (MRD) in the bone marrow and MS allowed the identification of double negative patients with a favorable PFS (median 3.33 years, 95% CI: 3.08-not reached) and no overall survival events. Our study provides strong evidence that MS is superior to conventional response monitoring, highlighting the potential of MS to become a new standard. Our data indicate that MS should be performed sequentially and combined with baseline disease features and MRD to improve its clinical value.Clinical Trials Register: EudraCT No. 2010-019173-16.
© 2022. The Author(s).
Conflict of interest statement
E.K.M.: Consulting or Advisory Role, Honoraria, Research Funding, and Travel Accommodations and Expenses—Bristol Myers Squibb/Celgene, GlaxoSmithKline, Janssen-Cilag, Sanofi, Stemline and Takeda. Oscar Berlanga is an employee of The Binding Site Group Ltd., UK. M.H.: Consulting or Advisory Role, Honoraria: Amgen, Bayer Vital, Celgene, Gilead, Glaxo Smith Kline, Jazz Pharmaceuticals, Novartis, Roche, Takeda. H.J.S.: Honoraria: AbbVie, Amgen, AstraZeneca, BMS, Celgene, Chugai, GSK, Janssen, Oncopeptides, Pfizer, Sanofi, Sebia, TAD, Takeda; Travel, accommodations, expenses: Amgen, BMS, Celgene, Janssen, and Sanofi. The remaining authors declare no conflict of interest.
Figures




References
-
- Murray DL, Puig N, Kristinsson S, Usmani SZ, Dispenzieri A, Bianchi G et al. Mass spectrometry for the evaluation of monoclonal proteins in multiple myeloma and related disorders: an International Myeloma Working Group Mass Spectrometry Committee Report. Blood Cancer J. 2021; 11. 10.1038/s41408-021-00408-4. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

