One-year breakthrough SARS-CoV-2 infection and correlates of protection in fully vaccinated hematological patients
- PMID: 36599843
- PMCID: PMC9812742
- DOI: 10.1038/s41408-022-00778-3
One-year breakthrough SARS-CoV-2 infection and correlates of protection in fully vaccinated hematological patients
Abstract
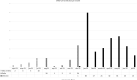
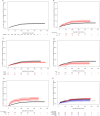
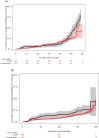
The long-term clinical efficacy of SARS-CoV-2 vaccines according to antibody response in immunosuppressed patients such as hematological patients has been little explored. A prospective multicenter registry-based cohort study conducted from December 2020 to July 2022 by the Spanish Transplant and Cell Therapy group, was used to analyze the relationship of antibody response over time after full vaccination (at 3-6 weeks, 3, 6 and 12 months) (2 doses) and of booster doses with breakthrough SARS-CoV-2 infection in 1551 patients with hematological disorders. At a median follow-up of 388 days after complete immunization, 266 out of 1551 (17%) developed breakthrough SARS-CoV-2 infection at median of 86 days (range 7-391) after full vaccination. The cumulative incidence was 18% [95% confidence interval (C.I.), 16-20%]. Multivariate analysis identified higher incidence in chronic lymphocytic leukemia patients (29%) and with the use of corticosteroids (24.5%), whereas female sex (15.5%) and more than 1 year after last therapy (14%) were associated with a lower incidence (p < 0.05 for all comparisons). Median antibody titers at different time points were significantly lower in breakthrough cases than in non-cases. A serological titer cut-off of 250 BAU/mL was predictive of breakthrough infection and its severity. SARS-CoV-2 infection-related mortality was encouragingly low (1.9%) in our series. Our study describes the incidence of and risk factors for COVID-19 breakthrough infections during the initial vaccination and booster doses in the 2021 to mid-2022 period. The level of antibody titers at any time after 2-dose vaccination is strongly linked with protection against both breakthrough infection and severe disease, even with the Omicron SARS-CoV-2 variant.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- García-Suárez J, de la Cruz J, Cedillo Á, Llamas P, Duarte R, Jiménez-Yuste V, et al. Impact of hematologic malignancy and type of cancer therapy on COVID-19 severity and mortality: lessons from a large population-based registry study. J Hematol Oncol. 2020;13:133. doi: 10.1186/s13045-020-00970-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

