Precision medicine: affording the successes of science
- PMID: 36599878
- PMCID: PMC9812011
- DOI: 10.1038/s41698-022-00343-y
Precision medicine: affording the successes of science
Abstract
Science has made remarkable advances in understanding the molecular basis of disease, generating new and effective rationally-designed treatments at an accelerating rate. Ironically, the successes of science is creating a crisis in the affordability of equitable health care. The COVID-19 pandemic underscores both the value of science in health care, and the apparently inevitable tension between health and the economy. Drug development in ever-smaller target populations is a critical component of the rising costs of care. For structural and historical reasons, drug development is inefficient and poorly integrated across the public and private sectors. We postulate an alternative, integrated model in which governments and industry share the risks and benefits of drug development. The Australian government recently announced support for a AU$185 million innovative multi-stakeholder public-private partnership model for sustainable precision oncology, accelerating biomarker-dependent drug development through integrating clinical trials into the standard of care.
© 2023. The Author(s).
Conflict of interest statement
DMT is the CEO of Omico, a non-profit precision oncology program. He has received research support as well as honoraria and speakers bureau from Astra Zeneca, Roche, Pfizer, Novartis, Eisai, Beigene, Seattle Genetics, Janssen, Bayer, Microba, InterVenn, Merck. VT is deputy CEO of Omico. The remaining authors declare no competing interests.
Figures
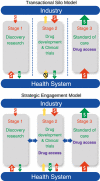



References
-
- Garrison LP, et al. Private sector risk-sharing agreements in the United States: trends, barriers, and prospects. Am. J. Manag Care. 2015;21:632–640. - PubMed
-
- Personalized Medicine Coalition. Personalized Medicine at FDA: A Progress & Outlook Report. https://www.personalizedmedicinecoalition.org/Userfiles/PMC-Corporate/fi..., (2018).
-
- Australian Government Department of Health and Aged Care. The Pharmaceutical Benefits Advisory Committee Guidelines. https://pbac.pbs.gov.au/information/about-the-guidelines.html, (2016).
Publication types
LinkOut - more resources
Full Text Sources

