Characterization of metabolic reprogramming by metabolomics in the oncocytic thyroid cancer cell line XTC.UC1
- PMID: 36599897
- PMCID: PMC9813134
- DOI: 10.1038/s41598-023-27461-2
Characterization of metabolic reprogramming by metabolomics in the oncocytic thyroid cancer cell line XTC.UC1
Abstract
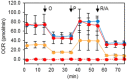
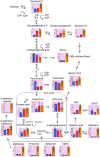
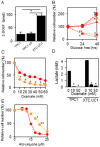
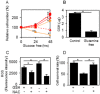
Oncocytic thyroid cancer is characterized by the aberrant accumulation of abnormal mitochondria in the cytoplasm and a defect in oxidative phosphorylation. We performed metabolomics analysis to compare metabolic reprogramming among the oncocytic and non-oncocytic thyroid cancer cell lines XTC.UC1 and TPC1, respectively, and a normal thyroid cell line Nthy-ori 3-1. We found that although XTC.UC1 cells exhibit higher glucose uptake than TPC1 cells, the glycolytic intermediates are not only utilized to generate end-products of glycolysis, but also diverted to branching pathways such as lipid metabolism and the serine synthesis pathway. Glutamine is preferentially used to produce glutathione to reduce oxidative stress in XTC.UC1 cells, rather than to generate α-ketoglutarate for anaplerotic flux into the TCA cycle. Thus, growth, survival and redox homeostasis of XTC.UC1 cells rely more on both glucose and glutamine than do TPC1 cells. Furthermore, XTC.UC1 cells contained higher amounts of intracellular amino acids which is due to higher expression of the amino acid transporter ASCT2 and enhanced autophagy, thus providing the building blocks for macromolecules and energy production. These metabolic alterations are required for oncocytic cancer cells to compensate their defective mitochondrial function and to alleviate excess oxidative stress.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials

