RBP-RNA interactions in the control of autoimmunity and autoinflammation
- PMID: 36599968
- PMCID: PMC9892603
- DOI: 10.1038/s41422-022-00752-5
RBP-RNA interactions in the control of autoimmunity and autoinflammation
Abstract
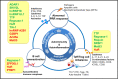
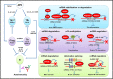
Autoimmunity and autoinflammation arise from aberrant immunological and inflammatory responses toward self-components, contributing to various autoimmune diseases and autoinflammatory diseases. RNA-binding proteins (RBPs) are essential for immune cell development and function, mainly via exerting post-transcriptional regulation of RNA metabolism and function. Functional dysregulation of RBPs and abnormities in RNA metabolism are closely associated with multiple autoimmune or autoinflammatory disorders. Distinct RBPs play critical roles in aberrant autoreactive inflammatory responses via orchestrating a complex regulatory network consisting of DNAs, RNAs and proteins within immune cells. In-depth characterizations of RBP-RNA interactomes during autoimmunity and autoinflammation will lead to a better understanding of autoimmune pathogenesis and facilitate the development of effective therapeutic strategies. In this review, we summarize and discuss the functions of RBP-RNA interactions in controlling aberrant autoimmune inflammation and their potential as biomarkers and therapeutic targets.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Sakaguchi S, et al. Regulatory T cells and human disease. Annu. Rev. Immunol. 2020;38:541–566. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

