Synaptic gradients transform object location to action
- PMID: 36599984
- PMCID: PMC9849133
- DOI: 10.1038/s41586-022-05562-8
Synaptic gradients transform object location to action
Erratum in
-
Publisher Correction: Synaptic gradients transform object location to action.Nature. 2023 Mar;615(7954):E26. doi: 10.1038/s41586-023-05930-y. Nature. 2023. PMID: 36914868 Free PMC article. No abstract available.
Abstract
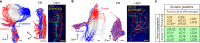
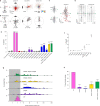
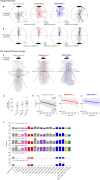
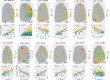
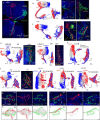
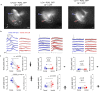
To survive, animals must convert sensory information into appropriate behaviours1,2. Vision is a common sense for locating ethologically relevant stimuli and guiding motor responses3-5. How circuitry converts object location in retinal coordinates to movement direction in body coordinates remains largely unknown. Here we show through behaviour, physiology, anatomy and connectomics in Drosophila that visuomotor transformation occurs by conversion of topographic maps formed by the dendrites of feature-detecting visual projection neurons (VPNs)6,7 into synaptic weight gradients of VPN outputs onto central brain neurons. We demonstrate how this gradient motif transforms the anteroposterior location of a visual looming stimulus into the fly's directional escape. Specifically, we discover that two neurons postsynaptic to a looming-responsive VPN type promote opposite takeoff directions. Opposite synaptic weight gradients onto these neurons from looming VPNs in different visual field regions convert localized looming threats into correctly oriented escapes. For a second looming-responsive VPN type, we demonstrate graded responses along the dorsoventral axis. We show that this synaptic gradient motif generalizes across all 20 primary VPN cell types and most often arises without VPN axon topography. Synaptic gradients may thus be a general mechanism for conveying spatial features of sensory information into directed motor outputs.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures












Comment in
-
A neural strategy for directional behaviour.Nature. 2023 Jan;613(7944):442-443. doi: 10.1038/d41586-022-04494-7. Nature. 2023. PMID: 36599995 No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

