The neuropeptide CGRP enters the macrophage cytosol to suppress the NLRP3 inflammasome during pulmonary infection
- PMID: 36600053
- PMCID: PMC9970963
- DOI: 10.1038/s41423-022-00968-w
The neuropeptide CGRP enters the macrophage cytosol to suppress the NLRP3 inflammasome during pulmonary infection
Abstract
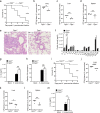
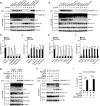
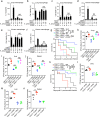
The NLRP3 inflammasome plays an essential role in resistance to bacterial infection. The nervous system secretes multiple neuropeptides affecting the nervous system as well as immune cells. The precise impact of the neuropeptide CGRP on NLRP3 inflammasome activation is still unclear. Here, we show that CGRP negatively regulates the antibacterial process of host cells. CGRP prevents NLRP3 inflammasome activation and reduces mature IL-1β secretion. Following NLRP3 inflammasome stimulation that triggers endosome leakage, CGRP internalized to endosomal compartments is released into the cell cytosol. Cytosolic CGRP binds directly to NLRP3 and dismantles the NLRP3-NEK7 complex, which is crucial for NLRP3 inflammasome activation. CGRP administration exacerbates bacterial infection, while the treatment with a CGRP antagonist has the opposite effect. Our study uncovers a unique role of CGRP in inhibiting inflammasome activation during infections, which might shed new light on antibacterial therapies in the future.
Keywords: CGRP; Inflammasome; NLRP3; Neuropeptide; Suppressor.
© 2022. The Author(s), under exclusive licence to CSI and USTC.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Charmoy M, Hurrell BP, Romano A, Lee SH, Ribeiro-Gomes F, Riteau N, et al. The Nlrp3 inflammasome, IL-1β, and neutrophil recruitment are required for susceptibility to a nonhealing strain of Leishmania major in C57BL/6 mice. Eur J Immunol. 2016;46:897–911. doi: 10.1002/eji.201546015. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

