Malignancy risk with tofacitinib versus TNF inhibitors in rheumatoid arthritis: results from the open-label, randomised controlled ORAL Surveillance trial
- PMID: 36600185
- PMCID: PMC9933177
- DOI: 10.1136/ard-2022-222543
Malignancy risk with tofacitinib versus TNF inhibitors in rheumatoid arthritis: results from the open-label, randomised controlled ORAL Surveillance trial
Abstract
Objectives: To evaluate malignancies and their associations with baseline risk factors and cardiovascular risk scores with tofacitinib versus tumour necrosis factor inhibitors (TNFi) in patients with rheumatoid arthritis (RA).
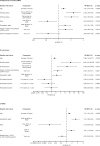
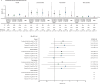
Methods: In an open-label, randomised controlled trial (ORAL Surveillance; NCT02092467), 4362 patients with RA aged ≥50 years with ≥1 additional cardiovascular risk factor received tofacitinib 5 (N=1455) or 10 mg two times per day (N=1456) or TNFi (N=1451). Incidence rates (IRs; patients with first events/100 patient-years) and HRs were calculated for adjudicated malignancies excluding non-melanoma skin cancer (NMSC), NMSC and subtypes. Post hoc analyses for malignancies excluding NMSC, lung cancer and NMSC included risk factors identified via simple/multivariable Cox models and IRs/HRs categorised by baseline risk factors, history of atherosclerotic cardiovascular disease (HxASCVD) and cardiovascular risk scores.
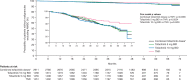
Results: IRs for malignancies excluding NMSC and NMSC were higher with tofacitinib (combined and individual doses) versus TNFi. Risk of lung cancer (most common subtype with tofacitinib) was higher with tofacitinib 10 mg two times per day versus TNFi. In the overall study population, the risk of malignancies excluding NMSC was similar between both tofacitinib doses and TNFi until month 18 and diverged from month 18 onwards (HR (95% CIs) for combined tofacitinib doses: 0.93 (0.53 to 1.62) from baseline to month 18 vs 1.93 (1.22 to 3.06) from month 18 onwards, interaction p=0.0469). Cox analyses identified baseline risk factors across treatment groups for malignancies excluding NMSC, lung cancer and NMSC; interaction analyses generally did not show statistical evidence of interaction between treatment groups and risk factors. HxASCVD or increasing cardiovascular risk scores were associated with higher malignancy IRs across treatments.
Conclusions: Risk of malignancies was increased with tofacitinib versus TNFi, and incidence was highest in patients with HxASCVD or increasing cardiovascular risk. This may be due to shared risk factors for cardiovascular risk and cancer.
Trial registration numbers: NCT02092467, NCT01262118, NCT01484561, NCT00147498, NCT00413660, NCT00550446, NCT00603512, NCT00687193, NCT01164579, NCT00976599, NCT01059864, NCT01359150, NCT02147587, NCT00960440, NCT00847613, NCT00814307, NCT00856544, NCT00853385, NCT01039688, NCT02281552, NCT02187055, NCT02831855, NCT00413699, NCT00661661.
Keywords: antirheumatic agents; arthritis, rheumatoid; cardiovascular diseases; tumor necrosis factor inhibitors.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JRC has received research/grant support from Amgen, CorEvitas, Crescendo Bio and Pfizer; and is a consultant for AbbVie, Amgen, Bristol-Myers Squibb, CorEvitas, Eli Lilly, Janssen, Myriad, Pfizer, Roche/Genentech and UCB. KY has received research/grant support from Astellas Pharma, Eisai Pharma, Eli Lilly Japan KK, GlaxoSmithKline, Mitsubishi Tanabe Pharma, MSD, Pfizer and Teijin Pharma; is a consultant for AbbVie, Asahi Kasei Pharma, Astellas Pharma, Eli Lilly Japan KK, Gilead GK, Japan Tobacco and Pfizer; and is part of speakers’ bureau for AbbVie, Actelion Pharmaceuticals Japan, Asahi Kasei Pharma, Astellas Pharma, AYUMI Pharma Co., Boehringer Ingelheim Japan, Bristol-Myers Squibb, Chugai Pharma, Daiichi Sankyo, Eli Lilly Japan KK, Eisai Pharma, Gilead GK, GlaxoSmithKline, Hisamitsu Pharma Co., Janssen, Japan Tobacco, Mitsubishi Tanabe Pharma, MSD, Nippon Kayaku, Nippon Shinyaku, Ono Pharma, Otsuka Pharma, Pfizer, Sanofi and Takeda. Y-HC has received research/grant support from AbbVie, Agnitio Science & Technology, Astellas Pharma, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai Pharma Taiwan, GlaxoSmithKline, Johnson & Johnson, MSD, National Yang-Ming University, Novartis, Pfizer, Roche, Sanofi, Taiwan Ministry of Science and Technology, Taiwan Department of Health and Welfare, Taichung Veterans General Hospital and UCB; and is a consultant for AbbVie, AstraZeneca, Astellas Pharma, Bristol-Myers Squibb, Chugai Pharma Taiwan, Inova Diagnostics, Eli Lilly, Johnson & Johnson, GlaxoSmithKline, MSD, Novartis, Roche, Sanofi, Thermo Fisher Scientific, UCB and United Biopharma. DLB served as a member of the Steering Committee for ORAL Surveillance, with funding from Pfizer paid to Brigham and Women’s Hospital. He is a member of the advisory board for: AngioWave, Bayer, Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, High Enroll, Janssen, Level Ex, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, Stasys; Board of Directors: AngioWave (stock options), Boston VA Research Institute, Bristol-Myers Squibb (stock), DRS.LINQ (stock options), High Enroll (stock), Society of Cardiovascular Patient Care, TobeSoft; Chair: Inaugural Chair, American Heart Association Quality Oversight Committee; Data Monitoring Committees: Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo; for the ABILITY-DM trial, funded by Concept Medical), Novartis, Population Health Research Institute; Rutgers University (for the NIH-funded MINT Trial); Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Oakstone CME (Course Director, Comprehensive Review of Interventional Cardiology), Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees), Wiley (steering committee); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Patent: Sotagliflozin (named on a patent for sotagliflozin assigned to Brigham and Women's Hospital who assigned to Lexicon; DLB/Brigham and Women's Hospital do not receive any income from this patent); Research Funding: Abbott, Acesion Pharma, Afimmune, Aker Biomarine, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, 89Bio; Royalties: Elsevier (Editor, Braunwald’s Heart Disease); Site Co-Investigator: Abbott, Biotronik, Boston Scientific, CSI, Endotronix, St. Jude Medical (now Abbott), Philips, SpectraWAVE, Svelte, Vascular Solutions; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Takeda. LMG, NS, CW, JW, SM and IV are shareholders and employees of Pfizer. CAC was a shareholder and employee of Pfizer at the time of this analysis. JJG-R has received research/grant support from AbbVie, MSD, Pfizer and Roche; is a consultant for Pfizer; and is part of speakers’ bureau for AbbVie, Biogen, Bristol-Myers Squibb, Janssen, MSD, Pfizer and Roche.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

