Patterns of toxicity burden for FDA-approved immune checkpoint inhibitors in the United States
- PMID: 36600271
- PMCID: PMC9814433
- DOI: 10.1186/s13046-022-02568-y
Patterns of toxicity burden for FDA-approved immune checkpoint inhibitors in the United States
Abstract
Background: Immune-related adverse events (irAEs) are a common phenomenon in cancer patients treated with immune checkpoint inhibitors (ICIs). Surprisingly, the toxicity burdens of these irAEs have not been illustrated clearly. In this study, we analyzed irAEs for seven FDA-approved ICIs in cancer treatment to show the pattern of toxicity burden among cancer patients.
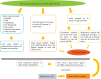
Methods: irAEs associated with seven FDA-approved ICIs, including three PD-1 inhibitors (cemiplimab, nivolumab and pembrolizumab), three PD-L1 inhibitors (atezolizumab, avelumab and durvalumab), and one CTLA-4 inhibitor (ipilimumab), were analyzed based on data from 149,303 reported cases (from January 1, 2015 to June 30, 2022) collected from the FDA Adverse Events Reporting System (FAERS) public dashboard. Proportions of serious irAEs and correlations with tumor type, age and sex were assessed via R package and GraphPad software.
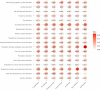
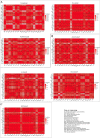
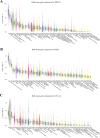
Results: irAEs related to anti-PD-1 ICIs required less hospital care resources compared with anti-PD-L1 and anti-CTLA-4 ICIs. Patients treated with pembrolizumab had relatively fewer serious cases. Treatment with ICIs led to the highest probability of serious irAEs in patients with lung cancer. 'Respiratory, thoracic and mediastinal disorders' and 'gastrointestinal disorders' were the two most common groups of disorders caused by the seven ICIs studied. 'Cardiac disorders' was the main type of disorders caused by these ICIs in cancer patients aged 65-85, while 'reproductive system and breast disease' was the main type of disorder in cancer patients aged 18-64. 'Respiratory, thoracic, mediastinal diseases' and 'reproductive system and breast diseases' were the main types of disorders associated with treatment with these ICIs in male and female patients, respectively.
Conclusion: Tissue and organ toxicities of ICIs are age and sex specific. There are risks of respiratory and urinary system toxicity in male patients and reproductive system toxicity in female patients treated with the ICIs studied. Future studies on the toxicity burden of ICIs should incorporate age and sex differences to better understand the relevance of ICI toxicity burden to human immune function to develop appropriate tumor immune and therapeutic intervention strategies.
Keywords: Adverse events; Immunotherapy; Therapeutic intervention; Toxicity burden; immune checkpoint inhibitors.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

