Interstitial pneumonitis associated with combined regimen of immunotherapy and conventional therapies-pharmacovigilance database analysis with real-world data validation
- PMID: 36600276
- PMCID: PMC9814324
- DOI: 10.1186/s12916-022-02713-6
Interstitial pneumonitis associated with combined regimen of immunotherapy and conventional therapies-pharmacovigilance database analysis with real-world data validation
Abstract
Background: Immune checkpoint inhibitor (ICI) therapy combined with conventional therapies is being broadly applied in non-small cell lung cancer (NSCLC) patients. However, the risk of interstitial pneumonitis (IP) following a combined regimen is incompletely characterized.
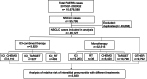
Methods: A total of 46,127 NSCLC patients were extracted for disproportionality analyses of IP from the Food and Drug Administration's Adverse Event Reporting System (FAERS) database. A total of 1108 NSCLC patients who received ICI treatment at Nanfang Hospital of Southern Medical University were collected and utilized for real-world validation.
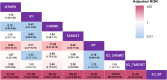
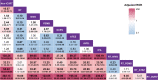
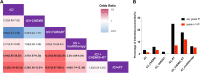
Results: Of the 46,127 patients with NSCLC, 3830 cases (8.3%; 95% confidence interval [CI], 8.05-8.56) developed IP. Multivariable logistic regression analyses revealed that the adjusted ROR of ICI combined with radiation (RT) was the highest (121.69; 95% CI, 83.60-184.96; P < 0.0001) among all therapies, while that of ICI combined with chemotherapy (CHEMO) or targeted therapy (TARGET) was 0.90 (95% CI, 0.78-1.04; P = 0.160) and 1.49 (95% CI, 0.95-2.23; P = 0.065), respectively, using ICI monotherapy as reference. Furthermore, analyses from our validation cohort of 1108 cases showed that the adjusted odds ratio of ICI combined with RT was the highest (12.25; 95% CI, 3.34-50.22; P < 0.01) among all the therapies, while that of ICI combined with CHEMO or TARGET was 2.32 (95% CI, 0.89-7.92; P = 0.12) and 0.66 (95% CI, 0.03-4.55; P = 0.71), respectively, using ICI monotherapy as reference.
Conclusions: Compared with ICI monotherapy, ICI combined with RT, rather than with CHEMO or TARGET, is associated with a higher risk of IP in NSCLC patients. Hence, patients receiving these treatments should be carefully monitored for IP.
Keywords: Conventional therapy; Immune checkpoint inhibitors; Interstitial pneumonitis; Non-small cell lung cancer; Radiation therapy.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

