Neuroblasts migration under control of reactive astrocyte-derived BDNF: a promising therapy in late neurogenesis after traumatic brain injury
- PMID: 36600294
- PMCID: PMC9814466
- DOI: 10.1186/s13287-022-03232-0
Neuroblasts migration under control of reactive astrocyte-derived BDNF: a promising therapy in late neurogenesis after traumatic brain injury
Abstract
Background: Traumatic brain injury (TBI) is a disease with high mortality and morbidity, which leads to severe neurological dysfunction. Neurogenesis has provided therapeutic options for treating TBI. Brain derived neurotrophic factor (BDNF) plays a key role in neuroblasts migration. We aimed to investigate to the key regulating principle of BDNF in endogenous neuroblasts migration in a mouse TBI model.
Methods: In this study, controlled cortical impact (CCI) mice (C57BL/6J) model was established to mimic TBI. The sham mice served as control. Immunofluorescence staining and enzyme-linked immunosorbent assay were performed on the CCI groups (day 1, 3, 7, 14 and 21 after CCI) and the sham group. All the data were analyzed with Student's t-test or one-way or two-way analysis of variance followed by Tukey's post hoc test.
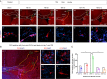
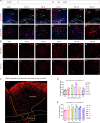
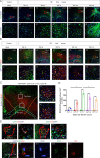
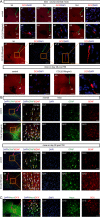
Results: Our results revealed that neuroblasts migration initiated as early as day 1, peaking at day 7, and persisted till day 21. The spatiotemporal profile of BDNF expression was similar to that of neuroblasts migration, and BDNF level following CCI was consistently higher in injured cortex than in subventricular zone (SVZ). Reactive astrocytes account for the major resource of BDNF along the migrating path, localized with neuroblasts in proximity. Moreover, injection of exogenous CC chemokine ligand 2 (CCL2), also known as monocyte chemoattractant protein-1, at random sites promoted neuroblasts migration and astrocytic BDNF expression in both normal and CCI mice (day 28). These provoked neuroblasts can also differentiate into mature neurons. CC chemokine ligand receptor 2 antagonist can restrain the neuroblasts migration after TBI.
Conclusions: Neuroblasts migrated along the activated astrocytic tunnel, directed by BDNF gradient between SVZ and injured cortex after TBI. CCL2 might be a key regulator in the above endogenous neuroblasts migration. Moreover, delayed CCL2 administration may provide a promising therapeutic strategy for late neurogenesis post-trauma.
Keywords: Brain-derived neurotrophic factor; CC chemokine ligand 2; Monocyte chemoattractant protein-1; Neuroblast; Neuronal migration; Traumatic brain injury.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

