S1PR1/S1PR3-YAP signaling and S1P-ALOX15 signaling contribute to an aggressive behavior in obesity-lymphoma
- PMID: 36600310
- PMCID: PMC9814427
- DOI: 10.1186/s13046-022-02589-7
S1PR1/S1PR3-YAP signaling and S1P-ALOX15 signaling contribute to an aggressive behavior in obesity-lymphoma
Abstract
Background: Excess body weight has been found to associate with an increased risk of lymphomas and some metabolic pathways are currently recognized in lymphomagenesis. Bioactive lipid metabolites such as sphingosine-1-phosphate (S1P) have been proposed to play an important role linking obesity and lymphomas. However, the underlying mechanism(s) of S1P signaling in obesity-lymphomagenesis have not been well addressed.
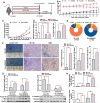
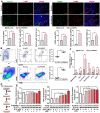
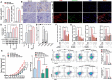
Methods: The gene expression of sphingosine kinase (SPHK), lymphoma prognosis, and S1P production were analyzed using Gene Expression Omnibus (GEO) and human lymphoma tissue array. Obesity-lymphoma mouse models and lymphoma cell lines were used to investigate the S1P/SPHK-YAP axis contributing to obesity-lymphomagenesis. By using the mouse models and a monocyte cell line, S1P-mediated polarization of macrophages in the tumor microenvironment were investigated.
Results: In human study, up-regulated S1P/SPHK1 was found in human lymphomas, while obesity negatively impacted progression-free survival and overall survival in lymphoma patients. In animal study, obesity-lymphoma mice showed an aggressive tumor growth pattern. Both in vivo and in vitro data suggested the existence of S1P-YAP axis in lymphoma cells, while the S1P-ALOX15 signaling mediated macrophage polarization towards TAMs exacerbated the lymphomagenesis. In addition, treatment with resveratrol in obesity-lymphoma mice showed profound effects of anti-lymphomagenesis, via down-regulating S1P-YAP axis and modulating polarization of macrophages.
Conclusion: S1P/S1PR initiated the feedback loops, whereby S1P-S1PR1/S1PR3-YAP signaling mediated lymphomagenesis contributing to tumor aggressive growth, while S1P-ALOX15 signaling mediated TAMs contributing to immunosuppressive microenvironment in obesity-lymphoma. S1P-targeted therapy could be potentially effective and immune-enhancive against obesity-lymphomagenesis.
Keywords: Free fatty acid; Lymphoma; Obesity; S1P/SPHK signaling; Tumor microenvironment.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Organization WH. 2022 https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight#....
-
- Lega IC, Lipscombe LL. Review: diabetes, obesity, and Cancer-pathophysiology and clinical implications. Endocr Rev. 2020;41(1). 10.1210/endrev/bnz014. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

