Utilisation, effectiveness, and safety of immediate postpartum intrauterine device insertion: a systematic literature review
- PMID: 36600467
- PMCID: PMC10176355
- DOI: 10.1136/bmjsrh-2022-201579
Utilisation, effectiveness, and safety of immediate postpartum intrauterine device insertion: a systematic literature review
Abstract
Background: Intrauterine devices (IUDs) are highly effective contraception. IUDs inserted directly following delivery provide immediate birth control and may decrease unintended pregnancies, including short-interval pregnancies, thereby mitigating health risks and associated economic burden.
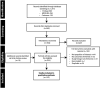
Methods: This systematic literature review included published global data on the utilisation, effectiveness, and safety of postpartum intrauterine devices (PPIUDs) of any type. English language articles indexed in MEDLINE, Embase, and Cochrane from January 2010-October 2021 were included.
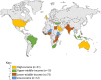
Results: 133 articles met the inclusion criteria (46% interventional studies; 54% observational; n=87 from lower-income countries; n=46 from higher-income countries). PPIUD use was low in higher-income countries (6/10 000 US deliveries in 2013-2016) and varied widely in lower-income countries (2%-46%). Across both higher- and lower-income countries, in most studies (79%), >80% of women with PPIUDs had an IUD in place by 3 months; at 6 and 12 months, 76% and 54% of included studies reported that >80% of women had an IUD in place; reason for discontinuation was infrequently reported. Pregnancies were rare (96 pregnancies across 12 191 women from 37 studies reporting data) and were generally unrelated to device failure, but rather occurred in women no longer using a PPIUD. Expulsions occurred mainly in the early outpatient period and ranged widely (within 3 months: 0-41%). Abnormal bleeding, infections, or perforations were rare.
Conclusions: PPIUDs are safe and effective. Long-term follow-up data are limited. Future research elucidating reasons underlying lack of PPIUD use is warranted.
Keywords: contraceptive effectiveness; intrauterine devices; long-acting reversible contraception; patient safety.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: EG-E reports personal fees from Xcenda LLC, during the conduct of the study. JL reports personal fees, non-financial support and other from Bayer US LLC, outside the submitted work. KRB, YW, NW, and FP are all employees of Bayer, which manufactures IUDs among its portfolio of products.
Figures
References
-
- Unintended pregnancy. Available: https://www.cdc.gov/reproductivehealth/contraception/unintendedpregnancy... [Accessed 23 Mar 2021].
-
- Our world in data, number of births per year, world. Available: https://ourworldindata.org/grapher/births-and-deaths-projected-to-2100?c... [Accessed 23 Mar 2021].
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
