TGFβ-derived immune modulatory vaccine: targeting the immunosuppressive and fibrotic tumor microenvironment in a murine model of pancreatic cancer
- PMID: 36600556
- PMCID: PMC9730419
- DOI: 10.1136/jitc-2022-005491
TGFβ-derived immune modulatory vaccine: targeting the immunosuppressive and fibrotic tumor microenvironment in a murine model of pancreatic cancer
Abstract
Background: Pancreatic ductal adenocarcinoma (PDAC) is associated with very poor survival, making it the third and fourth leading cause of all cancer-related deaths in the USA and European Union, respectively. The tumor microenvironment (TME) in PDAC is highly immunosuppressive and desmoplastic, which could explain the limited therapeutic effect of immunotherapy in PDAC. One of the key molecules that contributes to immunosuppression and fibrosis is transforming growth factor-β (TGFβ). The aim of this study was to target the immunosuppressive and fibrotic TME in PDAC using a novel immune modulatory vaccine with TGFβ-derived peptides in a murine model of pancreatic cancer.
Methods: C57BL/6 mice were subcutaneously inoculated with Pan02 PDAC cells. Mice were treated with TGFβ1-derived peptides (major histocompatibility complex (MHC)-I and MHC-II-restricted) adjuvanted with Montanide ISA 51VG. The presence of treatment-induced TGFβ-specific T cells was assessed by ELISpot (enzyme-linked immunospot). Changes in the immune infiltration and gene expression profile in tumor samples were characterized by flow cytometry, reverse transcription-quantitative PCR (RT-qPCR), and bulk RNA sequencing.
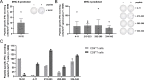
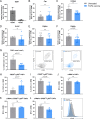
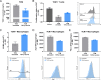
Results: Treatment with immunogenic TGFβ-derived peptides was safe and controlled tumor growth in Pan02 tumor-bearing mice. Enlargement of tumor-draining lymph nodes in vaccinated mice positively correlated to the control of tumor growth. Analysis of immune infiltration and gene expression in Pan02 tumors revealed that TGFβ-derived peptide vaccine increased the infiltration of CD8+ T cells and the intratumoral M1/M2 macrophage ratio, it increased the expression of genes involved in immune activation and immune response to tumors, and it reduced the expression of myofibroblast-like cancer-associated fibroblast (CAF)-related genes and genes encoding fibroblast-derived collagens. Finally, we confirmed that TGFβ-derived peptide vaccine actively modulated the TME, as the ability of T cells to proliferate was restored when exposed to tumor-conditioned media from vaccinated mice compared with media from untreated mice.
Conclusion: This study demonstrates the antitumor activity of TGFβ-derived multipeptide vaccination in a murine tumor model of PDAC. The data suggest that the vaccine targets immunosuppression and fibrosis in the TME by polarizing the cellular composition towards a more pro-inflammatory phenotype. Our findings support the feasibility and potential of TGFβ-derived peptide vaccination as a novel immunotherapeutic approach to target immunosuppression in the TME.
Keywords: Immunogenicity, Vaccine; Immunotherapy; Tumor Microenvironment; Vaccination.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: MHA has made an invention based on the use of transforming growth factor-β-derived peptides for vaccinations. A patent application directed to the invention is owned by the company IO Biotech ApS and lists MHA as the sole inventor. MHA is advisor and shareholder at IO Biotech. IL and EM are employees at IO Biotech. The additional authors declare no competing financial interests.
Figures



References
-
- Chen IM, Johansen JS, Theile S. Randomized phase II study of nivolumab with or without ipilimumab combined with stereotactic body radiotherapy for refractory metastatic pancreatic cancer (CheckPAC). J Clin Oncol 2022;1. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
