Circulating brain-derived extracellular vesicles expressing neuroinflammatory markers are associated with HIV-related neurocognitive impairment
- PMID: 36601110
- PMCID: PMC9806169
- DOI: 10.3389/fimmu.2022.1033712
Circulating brain-derived extracellular vesicles expressing neuroinflammatory markers are associated with HIV-related neurocognitive impairment
Abstract
Background: Neurocognitive impairment remains prevalent in people with HIV (PWH) despite long term virological suppression by antiretroviral therapy (ART) regimens. Systemic and neuro-inflammatory processes are suggested to contribute to the complex pathology leading to cognitive impairment in this population, yet the underlying mechanisms remain unresolved. Extracellular vesicles (EVs) play a central role in intracellular communication and have emerged as key modulators of immunological and inflammatory responses. In this report, we examined the impact of EVs in PWH experiencing cognitive deficits to determine their relevance in HIV associated neuropathology.
Methods: EV phenotypes were measured in plasma samples from 108 PWH with either cognitive impairment (CI, n=92) or normal cognition (NC, n=16) by flow cytometry. Matched cerebrospinal fluid (CSF)-derived EVs were similarly profiled from a subgroup of 84 individuals who underwent a lumbar puncture. Peripheral blood mononuclear cells were assayed by flow cytometry to measure monocyte frequencies in a subset of 32 individuals.
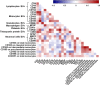
Results: Plasma-EVs expressing CD14, CD16, CD192, C195, and GFAP were significantly higher in HIV-infected individuals with cognitive impairment compared to individuals with normal cognition. Increased CSF-EVs expressing GFAP and CD200 were found in the cognitive impairment group compared to the normal cognition group. Frequencies of patrolling monocytes correlated with plasma-EVs expressing CD14, CD66b, MCSF, MAP2, and GFAP. Frequencies of CD195 expression on monocytes correlated positively with plasma-EVs expressing CD41a, CD62P, and CD63. Expression of CD163 on monocytes correlated positively with CSF-EVs expressing GFAP and CD200. Finally, the expression of CD192 on total monocytes correlated with CSF-EVs expressing CD200, CD62P, and CD63.
Conclusions: EVs expressing monocyte activation and neuronal markers associated with HIV associated cognitive impairment, suggesting that distinct EV subsets may serve as novel biomarkers of neuronal injury in HIV infection. Further circulating platelet EV levels were linked to monocyte activation indicating a potential novel interaction in the pathogenesis of HIV-related cognitive impairment.
Keywords: extracellular vesicles; human immunodeficiency virus; monocytes; neurocognitive impairment; neurons.
Copyright © 2022 de Menezes, Liu, Bowler, Giron, D’Antoni, Shikuma, Abdel-Mohsen, Ndhlovu and Norris.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- For the German Association of Neuro-AIDS und Neuro-Infectiology (DGNANI) Eggers C, Arendt G, Hahn K, Husstedt IW, Maschke M, et al. . HIV-1-associated neurocognitive disorder: Epidemiology, pathogenesis, diagnosis, and treatment. J Neurol (2017) 264(8):1715–27. doi: 10.1007/s00415-017-8503-2 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

