CLIC-01: Manufacture and distribution of non-cryopreserved CAR-T cells for patients with CD19 positive hematologic malignancies
- PMID: 36601119
- PMCID: PMC9806210
- DOI: 10.3389/fimmu.2022.1074740
CLIC-01: Manufacture and distribution of non-cryopreserved CAR-T cells for patients with CD19 positive hematologic malignancies
Abstract
Access to commercial CD19 CAR-T cells remains limited even in wealthy countries like Canada due to clinical, logistical, and financial barriers related to centrally manufactured products. We created a non-commercial academic platform for end-to-end manufacturing of CAR-T cells within Canada's publicly funded healthcare system. We report initial results from a single-arm, open-label study to determine the safety and efficacy of in-house manufactured CD19 CAR-T cells (entitled CLIC-1901) in participants with relapsed/refractory CD19 positive hematologic malignancies. Using a GMP compliant semi-automated, closed process on the Miltenyi Prodigy, T cells were transduced with lentiviral vector bearing a 4-1BB anti-CD19 CAR transgene and expanded. Participants underwent lymphodepletion with fludarabine and cyclophosphamide, followed by infusion of non-cryopreserved CAR-T cells. Thirty participants with non-Hodgkin's lymphoma (n=25) or acute lymphoblastic leukemia (n=5) were infused with CLIC-1901: 21 males (70%), median age 66 (range 18-75). Time from enrollment to CLIC-1901 infusion was a median of 20 days (range 15-48). The median CLIC-1901 dose infused was 2.3 × 106 CAR-T cells/kg (range 0.13-3.6 × 106/kg). Toxicity included ≥ grade 3 cytokine release syndrome (n=2) and neurotoxicity (n=1). Median follow-up was 6.5 months. Overall response rate at day 28 was 76.7%. Median progression-free and overall survival was 6 months (95%CI 3-not estimable) and 11 months (95% 6.6-not estimable), respectively. This is the first trial of in-house manufactured CAR-T cells in Canada and demonstrates that administering fresh CLIC-1901 product is fast, safe, and efficacious. Our experience may provide helpful guidance for other jurisdictions seeking to create feasible and sustainable CAR-T cell programs in research-oriented yet resource-constrained settings.
Clinical trial registration: https://clinicaltrials.gov/ct2/show/NCT03765177, identifier NCT03765177.
Keywords: CAR-T cells; hematologic malignancies; immune effector cells; in-house manufacturing; leukaemia; lymphoma; point-of-care manufacturing; prodigy.
Copyright © 2022 Kekre, Hay, Webb, Mallick, Balasundaram, Sigrist, Clement, Nielsen, Quizi, Yung, Brown, Dreolini, Waller, Smazynski, Gierc, Loveless, Clark, Dyer, Hogg, McCormick, Gignac, Bell, Chapman, Bond, Yong, Fung, Lockyer, Hodgson, Murphy, Subramanian, Wiebe, Yoganathan, Medynski, Vaillan, Black, McDiarmid, Kennah, Hamelin, Song, Narayanan, Rodrigo, Dupont, Hawrysh, Presseau, Thavorn, Lalu, Fergusson, Bell, Atkins, Nelson and Holt.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

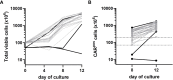

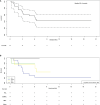
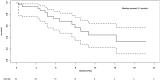


References
-
- Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. . Long-term safety and activity of axicabtagene ciloleucel in refractory large b-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol (2019) 20(1):31–42. doi: 10.1016/S1470-2045(18)30864-7 - DOI - PMC - PubMed
-
- Schuster SJ, Tam CS, Borchmann P, Worel N, McGuirk JP, Holte H, et al. . Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive b-cell lymphomas (JULIET): A multicentre, open-label, single-arm, phase 2 study. Lancet Oncol (2021) 22(10):1403–15. doi: 10.1016/S1470-2045(21)00375-2 - DOI - PubMed
-
- Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. . Lisocabtagene maraleucel for patients with relapsed or refractory large b-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet (2020) 396(10254):839–52. doi: 10.1016/S0140-6736(20)31366-0 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

