Recombinant actin-depolymerizing factor of the apicomplexan Neospora caninum (NcADF) is susceptible to oxidation
- PMID: 36601306
- PMCID: PMC9806845
- DOI: 10.3389/fcimb.2022.952720
Recombinant actin-depolymerizing factor of the apicomplexan Neospora caninum (NcADF) is susceptible to oxidation
Abstract
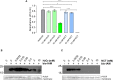
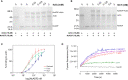
Neospora caninum is a member of Apicomplexa Phylum and the causative agent of neosporosis, a disease responsible for abortions in cattle. Apicomplexan parasites have a limited set of actin-binding proteins conducting the regulation of the dynamics of nonconventional actin. The parasite actin-based motility is implicated in the parasite invasion process in the host cell. Once no commercial strategy for the neosporosis control is available, the interference in the parasite actin function may result in novel drug targets. Actin-depolymerization factor (ADF) is a member of the ADF/cofilin family, primarily known for its function in actin severing and depolymerization. ADF/cofilins are versatile proteins modulated by different mechanisms, including reduction and oxidation. In apicomplexan parasites, the mechanisms involved in the modulation of ADF function are barely explored and the effects of oxidation in the protein are unknown so far. In this study, we used the oxidants N-chlorotaurine (NCT) and H2O2 to investigate the susceptibility of the recombinant N. caninum ADF (NcADF) to oxidation. After exposing the protein to either NCT or H2O2, the dimerization status and cysteine residue oxidation were determined. Also, the interference of NcADF oxidation in the interaction with actin was assessed. The treatment of the recombinant protein with oxidants reversibly induced the production of dimers, indicating that disulfide bonds between NcADF cysteine residues were formed. In addition, the exposure of NcADF to NCT resulted in more efficient oxidation of the cysteine residues compared to H2O2. Finally, the oxidation of NcADF by NCT reduced the ability of actin-binding and altered the function of NcADF in actin polymerization. Altogether, our results clearly show that recombinant NcADF is sensitive to redox conditions, indicating that the function of this protein in cellular processes involving actin dynamics may be modulated by oxidation.
Keywords: Apicomplexa; N-chlorotaurine; Neospora caninum; actin-binding protein; actin-depolymerizing factor (ADF); redox; taurine chloramine.
Copyright © 2022 Baroni, Abreu-Filho, Pereira, Nagl and Yatsuda.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Functional characterisation of the actin-depolymerising factor from the apicomplexan Neospora caninum (NcADF).Mol Biochem Parasitol. 2018 Sep;224:26-36. doi: 10.1016/j.molbiopara.2018.07.008. Epub 2018 Jul 21. Mol Biochem Parasitol. 2018. PMID: 30040977
-
Disassembly activity of actin-depolymerizing factor (ADF) is associated with distinct cellular processes in apicomplexan parasites.Mol Biol Cell. 2015 Sep 1;26(17):3001-12. doi: 10.1091/mbc.E14-10-1427. Epub 2015 Jul 8. Mol Biol Cell. 2015. PMID: 26157165 Free PMC article.
-
A mechanism for actin filament severing by malaria parasite actin depolymerizing factor 1 via a low affinity binding interface.J Biol Chem. 2014 Feb 14;289(7):4043-54. doi: 10.1074/jbc.M113.523365. Epub 2013 Dec 26. J Biol Chem. 2014. PMID: 24371134 Free PMC article.
-
ADF/cofilin: a functional node in cell biology.Trends Cell Biol. 2010 Apr;20(4):187-95. doi: 10.1016/j.tcb.2010.01.001. Epub 2010 Feb 3. Trends Cell Biol. 2010. PMID: 20133134 Free PMC article. Review.
-
Putting a new twist on actin: ADF/cofilins modulate actin dynamics.Trends Cell Biol. 1999 Sep;9(9):364-70. doi: 10.1016/s0962-8924(99)01619-0. Trends Cell Biol. 1999. PMID: 10461190 Review.
Cited by
-
ROS-dependent S-palmitoylation activates cleaved and intact gasdermin D.Nature. 2024 Jun;630(8016):437-446. doi: 10.1038/s41586-024-07373-5. Epub 2024 Apr 10. Nature. 2024. PMID: 38599239 Free PMC article.
-
Functional characterization of CpADF, an actin depolymerizing factor protein in Cryptosporidium parvum.Parasitol Res. 2023 Nov;122(11):2621-2630. doi: 10.1007/s00436-023-07960-x. Epub 2023 Sep 7. Parasitol Res. 2023. PMID: 37676305
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

