Trimer IgG and neutralising antibody response to COVID-19 mRNA vaccination in individuals with sarcoidosis
- PMID: 36601311
- PMCID: PMC9501840
- DOI: 10.1183/23120541.00025-2022
Trimer IgG and neutralising antibody response to COVID-19 mRNA vaccination in individuals with sarcoidosis
Abstract
Background: Individuals with sarcoidosis are at higher risk for infection owing to underlying disease pathogenesis and need for immunosuppressive treatment. Current knowledge as to how subjects with sarcoidosis respond to different forms of vaccination is limited. We examined quantitative and functional antibody response to COVID-19 vaccination in infection-naive subjects with and without sarcoidosis.
Methods: Our prospective cohort study recruited 14 subjects with biopsy-proven sarcoidosis and 27 age-sex matched controls who underwent a two-shot series of the BNT162b2 mRNA vaccine at the University of Illinois at Chicago. Baseline, 4-week and 6-month trimer spike protein IgG and neutralising antibody (nAb) titres were assessed. Correlation and multivariate regression analysis was conducted.
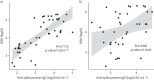
Results: Sarcoidosis subjects had a significant increase in short-term antibody production to a level comparable to controls; however, IgG titres significantly declined back to baseline levels by 6 months. Corresponding neutralising assays revealed robust nAb titres in sarcoidosis subjects that persisted at 6 months. A significant and strong correlation between IgG and nAb titres across all time points was observed in the control group. However within the sarcoidosis group, a significant but weak correlation between antibody levels was found. Overall, IgG levels were poor predictors of nAb titres at short- or long-term time points.
Conclusions: Sarcoidosis subjects exhibit nAb induced by the BNT162b2 mRNA SARS-CoV-2 vaccine at levels comparable to controls that persists at 6 months indicating conferred immunity. Trimer IgG levels are poor predictors of nAb in subjects with sarcoidosis. Studies of further antibody immunoglobulins and subtypes warrant investigation.
Copyright ©The authors 2023.
Conflict of interest statement
Conflict of interest: Richard M. Novak reports the following relationships outside the submitted work: grants or contracts received from Janssen; consulting fees received from Gilead and Viiv. The remaining authors have nothing to disclose.
Figures




Similar articles
-
Humoral immune response after different SARS-CoV-2 vaccination regimens.BMC Med. 2022 Jan 21;20(1):31. doi: 10.1186/s12916-021-02231-x. BMC Med. 2022. PMID: 35057798 Free PMC article.
-
BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers.Lancet Respir Med. 2021 Sep;9(9):999-1009. doi: 10.1016/S2213-2600(21)00220-4. Epub 2021 Jul 2. Lancet Respir Med. 2021. PMID: 34224675 Free PMC article.
-
Healthcare Workers in South Korea Maintain a SARS-CoV-2 Antibody Response Six Months After Receiving a Second Dose of the BNT162b2 mRNA Vaccine.Front Immunol. 2022 Jan 31;13:827306. doi: 10.3389/fimmu.2022.827306. eCollection 2022. Front Immunol. 2022. PMID: 35173736 Free PMC article.
-
COVID-19 lateral flow IgG seropositivity and serum neutralising antibody responses after primary and booster vaccinations in Chile: a cross-sectional study.Lancet Microbe. 2023 Mar;4(3):e149-e158. doi: 10.1016/S2666-5247(22)00290-7. Epub 2023 Jan 27. Lancet Microbe. 2023. PMID: 36716754 Free PMC article.
-
Correlates of protection against COVID-19 infection and intensity of symptomatic disease in vaccinated individuals exposed to SARS-CoV-2 in households in Israel (ICoFS): a prospective cohort study.Lancet Microbe. 2023 May;4(5):e309-e318. doi: 10.1016/S2666-5247(23)00012-5. Epub 2023 Mar 21. Lancet Microbe. 2023. PMID: 36963419 Free PMC article.
Cited by
-
Structural biology and public health response to biomedical threats.Struct Dyn. 2023 Jun 5;10(3):034701. doi: 10.1063/4.0000186. eCollection 2023 May. Struct Dyn. 2023. PMID: 37350851 Free PMC article.
-
Infectious Complications of Pulmonary Sarcoidosis.J Clin Med. 2024 Jan 7;13(2):342. doi: 10.3390/jcm13020342. J Clin Med. 2024. PMID: 38256476 Free PMC article. Review.
-
Initial behaviors and attitudes towards the COVID-19 vaccine in sarcoidosis patients: results of a self-reporting questionnaire.Sarcoidosis Vasc Diffuse Lung Dis. 2023 Jun 29;40(2):e2023012. doi: 10.36141/svdld.v40i2.14388. Sarcoidosis Vasc Diffuse Lung Dis. 2023. PMID: 37382069 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
