The des-Arg9-bradykinin/B1R axis: Hepatic damage in COVID-19
- PMID: 36601349
- PMCID: PMC9806358
- DOI: 10.3389/fphys.2022.1080837
The des-Arg9-bradykinin/B1R axis: Hepatic damage in COVID-19
Abstract
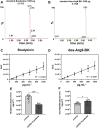
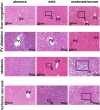
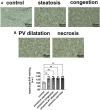
Patients infected by the SARS-CoV-2 virus are commonly diagnosed with threatening liver conditions associated with drug-induced therapies and systemic viral action. RNA-Seq data from cells in bronchoalveolar lavage fluid from COVID-19 patients have pointed out dysregulation of kallikrein-kinin and renin-angiotensin systems as a possible mechanism that triggers multi-organ damage away from the leading site of virus infection. Therefore, we measured the plasma concentration of biologically active peptides from the kallikrein-kinin system, bradykinin and des-Arg9-bradykinin, and liver expression of its proinflammatory axis, bradykinin 1 receptor (B1R). We measured the plasma concentration of bradykinin and des-Arg9-bradykinin of 20 virologically confirmed COVID-19 patients using a liquid chromatography-tandem mass spectrometry-based methodology. The expression of B1R was evaluated by immunohistochemistry from post-mortem liver specimens of 27 COVID-19 individuals. We found a significantly higher blood level of des-Arg9-bradykinin and a lower bradykinin concentration in patients with COVID-19 compared to a healthy, uninfected control group. We also observed increased B1R expression levels in hepatic tissues of patients with COVID-19 under all hepatic injuries analyzed (liver congestion, portal vein dilation, steatosis, and ischemic necrosis). Our data indicate that des-Arg9-bradykinin/B1R is associated with the acute hepatic dysfunction induced by the SARS-CoV-2 virus infection in the pathogenesis of COVID-19.
Keywords: COVID-19; SARS-cov-2; bradykinin; bradykinin 1 receptor; contact system; hepatic damage; kinin-kallikrein system; renin-angiotensin system.
Copyright © 2022 Mendes, Do Nascimento, Marazzi-Diniz, Da Silveira, Itaborahy, Viana, Silva, Santana, Pinto, Dutra, Lacerda, Araujo, Wanderley, Vidigal, Diniz, Verano-Braga, Santos and Leite.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Azinheira Nobrega Cruz N., Gonçalves de Oliveira L. C., Tedesco Silva Junior H., Osmar Medina Pestana J., Casarini D. E. (2021). Angiotensin-converting enzyme 2 in the pathogenesis of renal abnormalities observed in COVID-19 patients. Front. Physiol. 12, 700220. 10.3389/fphys.2021.700220 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

