Acanthamoeba spp. aggregate and encyst on contact lens material increasing resistance to disinfection
- PMID: 36601401
- PMCID: PMC9806144
- DOI: 10.3389/fmicb.2022.1089092
Acanthamoeba spp. aggregate and encyst on contact lens material increasing resistance to disinfection
Abstract
Introduction: Acanthamoeba keratitis is often caused when Acanthamoeba contaminate contact lenses and infect the cornea. Acanthamoeba is pervasive in the environment as a motile, foraging trophozoite or biocide-resistant and persistent cyst. As contact lens contamination is a potential first step in infection, we studied Acanthamoeba's behavior and interactions on different contact lens materials. We hypothesized that contact lenses may induce aggregation, which is a precursor to encystment, and that aggregated encystment would be more difficult to disinfect than motile trophozoites.
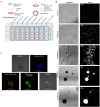
Methods: Six clinically and/or scientifically relevant strains of Acanthamoeba (ATCC 30010, ATCC 30461, ATCC 50370, ATCC 50702, ATCC 50703, and ATCC PRA-115) were investigated on seven different common silicone hydrogel contact lenses, and a no-lens control, for aggregation and encystment for 72 h. Cell count and size were used to determine aggregation, and fluorescent staining was used to understand encystment. RNA seq was performed to describe the genome of Acanthamoeba which was individually motile or aggregated on different lens materials. Disinfection efficacy using three common multi-purpose solutions was calculated to describe the potential disinfection resistance of trophozoites, individual cysts, or spheroids.
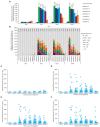
Results: Acanthamoeba trophozoites of all strains examined demonstrated significantly more aggregation on specific contact lens materials than others, or the no-lens control. Fluorescent staining demonstrated encystment in as little as 4 hours on contact lens materials, which is substantially faster than previously reported in natural or laboratory settings. Gene expression profiles corroborated encystment, with significantly differentially expressed pathways involving actin arrangement and membrane complexes. High disinfection resistance of cysts and spheroids with multi-purpose solutions was observed.
Discussion: Aggregation/encystment is a protective mechanism which may enable Acanthamoeba to be more disinfection resistant than individual trophozoites. This study demonstrates that some contact lens materials promote Acanthamoeba aggregation and encystment, and Acanthamoeba spheroids obstruct multi-purpose solutions from disinfecting Acanthamoeba.
Keywords: Acanthamoeba; aggregate; contact lens; contact lens solution; cyst; spheroid.
Copyright © 2022 Campolo, Pifer, Walters, Thomas, Miller, Harris, King, Rice, Shannon, Patterson and Crary.
Conflict of interest statement
The funder was not involved in the study design, collection, analysis, interpretation of data, or the writing of this article. The funder approved the decision to submit for publication. All authors except CR are employees of Alcon Research. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures









Similar articles
-
Disinfection efficacy and encystment rate of soft contact lens multipurpose solutions against Acanthamoeba.Eye Contact Lens. 2010 Jan;36(1):26-32. doi: 10.1097/ICL.0b013e3181c6e184. Eye Contact Lens. 2010. PMID: 20009947
-
Effects of multipurpose solutions on the viability and encystment of acanthamoeba determined by flow cytometry.Eye Contact Lens. 2013 May;39(3):228-33. doi: 10.1097/ICL.0b013e31828af147. Eye Contact Lens. 2013. PMID: 23584044
-
Development of standardized methods for assessing biocidal efficacy of contact lens care solutions against Acanthamoeba trophozoites and cysts.Invest Ophthalmol Vis Sci. 2013 Jul 5;54(7):4527-37. doi: 10.1167/iovs.13-11927. Invest Ophthalmol Vis Sci. 2013. PMID: 23745008
-
Acanthamoeba keratitis: a review of biology, pathophysiology and epidemiology.Ophthalmic Physiol Opt. 2021 Jan;41(1):116-135. doi: 10.1111/opo.12752. Epub 2020 Oct 29. Ophthalmic Physiol Opt. 2021. PMID: 33119189 Review.
-
Acanthamoeba: a review of its potential to cause keratitis, current lens care solution disinfection standards and methodologies, and strategies to reduce patient risk.Eye Contact Lens. 2008 Sep;34(5):247-53. doi: 10.1097/ICL.0b013e31817e7d83. Eye Contact Lens. 2008. PMID: 18779663 Review.
Cited by
-
New Frontiers in Acanthamoeba Keratitis Diagnosis and Management.Biology (Basel). 2023 Dec 5;12(12):1489. doi: 10.3390/biology12121489. Biology (Basel). 2023. PMID: 38132315 Free PMC article. Review.
-
Targets for the diagnosis of Acanthamoeba eye infections include four cyst wall proteins and the mannose-binding domain of the trophozoite mannose-binding protein.mSphere. 2025 Mar 25;10(3):e0094824. doi: 10.1128/msphere.00948-24. Epub 2025 Mar 4. mSphere. 2025. PMID: 40035521 Free PMC article.
-
Biological characteristics and pathogenicity of Acanthamoeba.Front Microbiol. 2023 Apr 5;14:1147077. doi: 10.3389/fmicb.2023.1147077. eCollection 2023. Front Microbiol. 2023. PMID: 37089530 Free PMC article. Review.
-
Time-Lapse Tracking of Acanthamoeba castellanii Trophozoite Activity During Exposure to Contact Lens Disinfectants.Invest Ophthalmol Vis Sci. 2025 Aug 1;66(11):50. doi: 10.1167/iovs.66.11.50. Invest Ophthalmol Vis Sci. 2025. PMID: 40844809 Free PMC article.
-
Complete Recovery of Acanthamoeba Motility among Surviving Organisms after Contact Lens Care Disinfection.Microorganisms. 2023 Jan 23;11(2):299. doi: 10.3390/microorganisms11020299. Microorganisms. 2023. PMID: 36838263 Free PMC article.
References
-
- Acanthamoeba polyphaga (Pushkarew) . (2019). Encyclopedia of life. Available at: https://eol.org/pages/593787 (Accessed October 6, 2022).
-
- Antonelli A., Favuzza E., Galano A., Montalbano Di Filippo M., Ciccone N., Berrilli F., et al. . (2018). Regional spread of contact lens-related Acanthamoeba keratitis in Italy. New Microbiol. 41, 83–85. PMID: - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

