Formulation of metoclopramide HCl gastroretentive film and in vitro- in silico prediction using Gastroplus® PBPK software
- PMID: 36601510
- PMCID: PMC9805977
- DOI: 10.1016/j.jsps.2022.10.011
Formulation of metoclopramide HCl gastroretentive film and in vitro- in silico prediction using Gastroplus® PBPK software
Abstract
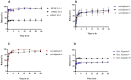
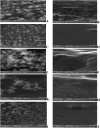
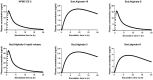
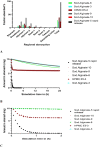
The new trends in pharmaceutical studies focus on targeting drug delivery and computer software that help in the body environment simulation, such as Gastroplus® software. The interest of this study is to prepare a gastroretentive film of metoclopramide HCl (MTC) that was followed by applying the in silico approach to estimate the in vivo prepared formulations. The films were prepared from HPMC E5 and sodium alginate polymers as primary polymers with the aid of secondary polymers. The sodium alginate high proportions films showed instant and long floating duration reaching 24 h but with variable folding endurance. Moreover, sodium alginate films with their secondary polymers carbopol and HPMC E5 slowed the release of MTC. The floating and slow-release patterns assessed the gastroretentive properties of sodium alginate films and were further examined by a mucoadhesive study that guaranteed mucosal adhesion, and the film's FESEM images showed similar top morphology, but different side view structures. Last, the pharmacokinetic profile of selected films that approached the gastroretentive properties was in silico predicted depending on in vitro release study and floating duration employing the physiological-based pharmacokinetic model in Gastroplus® software. The model determines this prediction found successfully of intravenous and immediate oral release tablets (10 and 30 mg) of MTC. The simulation showed a high amount of MTC retained for long periods in the stomach to Sod.Alginate-3, Sod.Alginate-8, and Sod.Alginate-10 films (films of secondary polymers carbopol and HPMC E5) aid in reaching the optimum site of absorption jejunum 1 due to the slow MTC release.
Keywords: Gastroplus®; Gastroretentive films; Metoclopramide HCl; PBPK.
© 2022 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Development of sustained release floating drug delivery system for norfloxacin: in vitro and in vivo evaluation.PDA J Pharm Sci Technol. 2011 May-Jun;65(3):198-206. doi: 10.5731/pdajpst.2011.00685. PDA J Pharm Sci Technol. 2011. PMID: 22293231
-
Physiological relevant in vitro evaluation of polymer coats for gastroretentive floating tablets.Eur J Pharm Biopharm. 2014 Nov;88(3):778-86. doi: 10.1016/j.ejpb.2014.07.009. Epub 2014 Jul 31. Eur J Pharm Biopharm. 2014. PMID: 25086221
-
Formulation, release characteristics, and bioavailability study of gastroretentive floating matrix tablet and floating raft system of Mebeverine HCl.Drug Des Devel Ther. 2017 Apr 3;11:1081-1093. doi: 10.2147/DDDT.S131936. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 28435220 Free PMC article.
-
DDSolver Software Application for Quantitative Analysis of In vitro Drug Release Behavior of the Gastroretentive Floating Tablets Combined with Radiological Study in Rabbits.Curr Drug Deliv. 2022 Aug 6;19(9):949-965. doi: 10.2174/1567201819666220304203014. Curr Drug Deliv. 2022. PMID: 35249487
-
SeDeM tool-driven full factorial design for osmotic drug delivery of tramadol HCl: Formulation development, physicochemical evaluation, and in-silico PBPK modeling for predictive pharmacokinetic evaluation using GastroPlus™.Front Pharmacol. 2022 Oct 7;13:974715. doi: 10.3389/fphar.2022.974715. eCollection 2022. Front Pharmacol. 2022. PMID: 36278217 Free PMC article.
Cited by
-
Advances in Modeling Approaches for Oral Drug Delivery: Artificial Intelligence, Physiologically-Based Pharmacokinetics, and First-Principles Models.Pharmaceutics. 2024 Jul 24;16(8):978. doi: 10.3390/pharmaceutics16080978. Pharmaceutics. 2024. PMID: 39204323 Free PMC article. Review.
References
-
- Anupam P., Ashwani M., Praveen M. Formulation and evaluation of gastroretentive mucoadhesive film of captopril. Pharmacia. 2013
-
- Bhardwaj P., Singh R., Swarup A. Development and characterization of newer floating film bearing 5-fluorouracil as a model drug. J. Drug Delivery Sci. Technol. 2014;24(5):486–490. doi: 10.1016/S1773-2247(14)50092-5. - DOI
LinkOut - more resources
Full Text Sources

