Baseline Characteristics of Pediatric Patients With Heart Failure Due to Systemic Left Ventricular Systolic Dysfunction in the PANORAMA-HF Trial
- PMID: 36601956
- PMCID: PMC10022669
- DOI: 10.1161/CIRCHEARTFAILURE.122.009816
Baseline Characteristics of Pediatric Patients With Heart Failure Due to Systemic Left Ventricular Systolic Dysfunction in the PANORAMA-HF Trial
Abstract
Background: Sacubitril/valsartan has been approved for the management of heart failure (HF) with reduced ejection fraction in adults. PANORAMA-HF trial (Prospective Trial to Assess the Angiotensin Receptor Blocker Neprilysin Inhibitor LCZ696 Versus Angiotensin-Converting Enzyme Inhibitor for the Medical Treatment of Pediatric HF) investigated its effects on clinical outcomes in pediatric patients with HF.
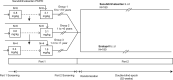
Methods: PANORAMA-HF is a multicenter, Phase II/III study using an adaptive, seamless, 2-part design. The study aimed to evaluate the pharmacokinetics and pharmacodynamics of single doses of sacubitril/valsartan (Part 1), and the efficacy and safety of sacubitril/valsartan versus enalapril administered twice daily for 52 weeks (Part 2) in pediatric patients with HF due to left ventricular systolic dysfunction with biventricular heart physiology. An innovative trial design using a novel global rank assessment of severity was employed. For analysis, eligible patients were stratified into 3 age groups (Group 1, 6 to <18 years; Group 2a, 2 to <6 years; and Group 3a, 1 month to <2 years) and functional classification (New York Heart Association/Ross class I/II and III/IV).
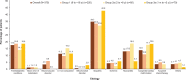
Results: We report the key demographic, baseline, and clinical characteristics of 375 pediatric patients randomized to receive the study medication. The mean age for patients in Groups 1, 2a, and 3a was 12.2, 3.2, and 1.3 years, respectively. About 70% of patients had a prior HF hospitalization, 85% had New York Heart Association/Ross class I/II HF, and ≈8% were angiotensin-converting enzyme inhibitor/angiotensin receptor blocker naïve.
Conclusions: Compared to other pediatric HF studies, PANORAMA-HF recruited a relatively homogeneous pediatric HF population across 3 age groups, enabling a more robust evaluation of pharmacokinetics/pharmacodynamics and efficacy/safety of sacubitril/valsartan. Most patients had mildly symptomatic HF at baseline.
Registration: URL: https://www.
Clinicaltrials: gov; Unique identifier: NCT02678312.
Keywords: Entresto; angiotensin receptor antagonist; children; clinical trial; heart failure; neprilysin; ventricular dysfunction.
Conflict of interest statement
Dr Shaddy is a consultant for Novartis, Bayer, and Bristol Myers Squibb. Dr Burch and P. Kantor are members of the PANORAMA-HF Executive Committee. S. Solar-Yohay and M. Kocun are employees of Novartis Pharmaceuticals, East Hanover, NJ. Dr Garito is an employee of Novartis Pharma AG, Basel, Switzerland. Dr Zhang is an employee of Novartis Pharma, Shanghai, China. Dr Bonnet is a consultant for Janssen-Janssen, Novartis, and Acceleron.
Figures
References
-
- Rossano JW, Kim JJ, Decker JA, Price JF, Zafar F, Graves DE, Morales DL, Heinle JS, Bozkurt B, Towbin JA, et al. Prevalence, morbidity, and mortality of heart failure-related hospitalizations in children in the United States: a population-based study. J Card Fail. 2012;18:459–470. doi: 10.1016/j.cardfail.2012.03.001 - PubMed
-
- Mejia EJ, O’Connor MJ, Lin KY, Song L, Griffis H, Mascio CE, Shamszad P, Donoghue A, Ravishankar C, Shaddy RE, et al. Characteristics and outcomes of pediatric heart failure-related emergency department visits in the united states: A population-based study. J Pediatr. 2018;193:114–118.e3. doi: 10.1016/j.jpeds.2017.10.009 - PubMed
-
- Shaddy RE, George AT, Jaecklin T, Lochlainn EN, Thakur L, Agrawal R, Solar-Yohay S, Chen F, Rossano JW, Severin T, et al. Systematic literature review on the incidence and prevalence of heart failure in children and adolescents. Pediatr Cardiol. 2018;39:415–436. doi: 10.1007/s00246-017-1787-2 - PMC - PubMed
-
- Masarone D, Valente F, Rubino M, Vastarella R, Gravino R, Rea A, Russo MG, Pacileo G, Limongelli G. Pediatric heart failure: A practical guide to diagnosis and management. Pediatr Neonatol. 2017;58:303–312. doi: 10.1016/j.pedneo.2017.01.001 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

