In Vitro Activity of Ceftibuten-Avibactam against β-Lactamase-Positive Enterobacterales from the ATLAS Global Surveillance Program
- PMID: 36602322
- PMCID: PMC9872606
- DOI: 10.1128/aac.01346-22
In Vitro Activity of Ceftibuten-Avibactam against β-Lactamase-Positive Enterobacterales from the ATLAS Global Surveillance Program
Abstract
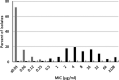
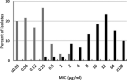
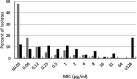
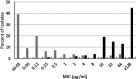
Ceftibuten is an established, oral, third-generation cephalosporin in early clinical development in combination with an oral prodrug of avibactam for the treatment of complicated urinary tract infections, including acute pyelonephritis. We evaluated the in vitro activity of ceftibuten-avibactam against 1,165 Enterobacterales isolates selected from the 2016-2020 ATLAS global surveillance program based upon their β-lactamase genotype, β-lactam-susceptible phenotype, species identification, and specimen source (95.8% urine). MICs were determined by CLSI broth microdilution. Avibactam was tested at a fixed concentration of 4 μg/mL. Molecular methods were used to identify β-lactamase genes. Ceftibuten-avibactam inhibited 90% (MIC90) of ESBL-producing (n = 645), KPC-producing (n = 60), chromosomal AmpC-positive (n = 100), OXA-48-like-producing (n = 50), and acquired AmpC-producing (n = 110) isolates at concentrations of 0.12, 0.5, 1, 2, and 4 μg/mL, respectively. At concentrations of ≤1 and ≤8 μg/mL, ceftibuten-avibactam inhibited 98.4 and 99.2% of ESBL-positive isolates; 96.7 and 100% of KPC-positive isolates; 91.0 and 99.0% of chromosomal AmpC-positive isolates; 86.0 and 96.0% of OXA-48-like-positive isolates; and 85.5 and 91.8% of acquired AmpC-positive isolates. Against ESBL-producing, KPC-producing, chromosomal AmpC-positive, OXA-48-like-producing, and acquired AmpC-producing isolates, ceftibuten-avibactam was 256-, 128-, >64-, >32-, and > 16-fold more potent than ceftibuten alone. The potency of ceftibuten-avibactam was 4-fold greater than ceftazidime-avibactam against ESBL-producing (ceftibuten-avibactam MIC90, 0.12 μg/mL; ceftazidime-avibactam MIC90, 0.5 μg/mL) and KPC-producing (0.5 μg/mL; 2 μg/mL) isolates, equivalent to ceftazidime-avibactam (MIC90, 2 μg/mL) against OXA-48-like-producing isolates, 2-fold less active than ceftazidime-avibactam (1 μg/mL; 0.5 μg/mL) against chromosomal AmpC-positive isolates, and 4-fold less active than ceftazidime-avibactam (4 μg/mL; 1 μg/mL) against acquired AmpC-producing isolates. Continued development of ceftibuten-avibactam appears justified.
Keywords: Enterobacterales; avibactam; ceftibuten; oral therapy; urinary tract infection.
Conflict of interest statement
The authors declare a conflict of interest. J.A.K. is a consultant to IHMA and does not have a personal financial interest in the sponsor of this manuscript (Pfizer). M.A.H. and D.F.S. are employees of IHMA, who were paid consultants to Pfizer in connection with the development of this manuscript, and do not have a personal financial interest in the sponsor of this paper (Pfizer). G.G.S. is an employee of Pfizer.
Figures


References
-
- World Health Organization. 2020. Target product profiles for oral therapy of urinary tract infections. https://apps.who.int/iris/bitstream/handle/10665/332250/9789240003873-en.... Accessed 1 October 2022.
-
- World Health Organization. 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-E.... Accessed 1 October 2022.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

