Specific Disruption of Ras2 CAAX Proteolysis Alters Its Localization and Function
- PMID: 36602340
- PMCID: PMC9927470
- DOI: 10.1128/spectrum.02692-22
Specific Disruption of Ras2 CAAX Proteolysis Alters Its Localization and Function
Abstract
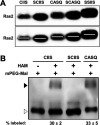
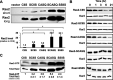
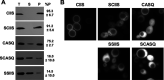
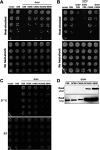
Many CAAX proteins, such as Ras GTPase, undergo a series of posttranslational modifications at their carboxyl terminus (i.e., cysteine prenylation, endoproteolysis of AAX, and carboxylmethylation). Some CAAX proteins, however, undergo prenylation-only modification, such as Saccharomyces cerevisiae Hsp40 Ydj1. We previously observed that altering the CAAX motif of Ydj1 from prenylation-only to canonical resulted in altered Ydj1 function and localization. Here, we investigated the effects of a reciprocal change that altered the well-characterized canonical CAAX motif of S. cerevisiae Ras2 to prenylation-only. We observed that the type of CAAX motif impacted Ras2 protein levels, localization, and function. Moreover, we observed that using a prenylation-only sequence to stage hyperactive Ras2-G19V as a farnesylated and nonproteolyzed intermediate resulted in a different phenotype relative to staging by a genetic RCE1 deletion strategy that simultaneously affected many CAAX proteins. These findings suggested that a prenylation-only CAAX motif is useful for probing the specific impact of CAAX proteolysis on Ras2 under conditions where other CAAX proteins are normally modified. We propose that our strategy could be easily applied to a wide range of CAAX proteins for examining the specific impact of CAAX proteolysis on their functions. IMPORTANCE CAAX proteins are subject to multiple posttranslational modifications: cysteine prenylation, CAAX proteolysis, and carboxylmethylation. For investigations of CAAX proteolysis, this study took the novel approach of using a proteolysis-resistant CAAX sequence to stage Saccharomyces cerevisiae Ras2 GTPase in a farnesylated and nonproteolyzed state. Our approach specifically limited the effects of disrupting CAAX proteolysis to Ras2. This represented an improvement over previous methods where CAAX proteolysis was inhibited by gene knockout, small interfering RNA knockdown, or biochemical inhibition of the Rce1 CAAX protease, which can lead to pleiotropic and unclear attribution of effects due to the action of Rce1 on multiple CAAX proteins. Our approach yielded results that demonstrated specific impacts of CAAX proteolysis on the function, localization, and other properties of Ras2, highlighting the utility of this approach for investigating the impact of CAAX proteolysis in other protein contexts.
Keywords: CAAX pathway; CAAX proteolysis; Ras; Ras signaling; Ras2; Rce1; lipidation; palmitoylation; proteolysis; shunt pathway; yeast.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Schmidt WK, Tam A, Fujimura-Kamada K, Michaelis S. 1998. Endoplasmic reticulum membrane localization of Rce1p and Ste24p, yeast proteases involved in carboxyl-terminal CAAX protein processing and amino-terminal a-factor cleavage. Proc Natl Acad Sci USA 95:11175–11180. doi:10.1073/pnas.95.19.11175. - DOI - PMC - PubMed
-
- Mitchell DA, Hamel LD, Ishizuka K, Mitchell G, Schaefer LM, Deschenes RJ. 2012. The Erf4 subunit of the yeast Ras palmitoyl acyltransferase is required for stability of the acyl-Erf2 intermediate and palmitoyl transfer to a Ras2 substrate. J Biol Chem 287:34337–34348. doi:10.1074/jbc.M112.379297. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

